A mouse model of binge alcohol consumption and Burkholderia infection
- PMID: 30485380
- PMCID: PMC6261616
- DOI: 10.1371/journal.pone.0208061
A mouse model of binge alcohol consumption and Burkholderia infection
Abstract
Background: Binge drinking, an increasingly common form of alcohol consumption, is associated with increased mortality and morbidity; yet, its effects on the immune system's ability to defend against infectious agents are poorly understood. Burkholderia pseudomallei, the causative agent of melioidosis can occur in healthy humans, yet binge alcohol use is progressively being recognized as a major risk factor. Although our previous studies demonstrated that binge alcohol exposure results in reduced alveolar macrophage function and increased Burkholderia virulence in vitro, no experimental studies have investigated the outcomes of binge alcohol on Burkholderia spp. infection in vivo.
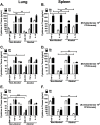
Principal findings: In this study, we used the close genetic relatives of B. pseudomallei, B. thailandensis E264 and B. vietnamiensis, as useful BSL-2 model systems. Eight-week-old female C57BL/6 mice were administered alcohol comparable to human binge drinking episodes (4.4 g/kg) or PBS intraperitoneally 30 min before a non-lethal intranasal infection. In an initial B. thailandensis infection (3 x 105), bacteria accumulated in the lungs and disseminated to the spleen in alcohol administered mice only, compared with PBS treated mice at 24 h PI. The greatest bacterial load occurred with B. vietnamiensis (1 x 106) in lungs, spleen, and brain tissue by 72 h PI. Pulmonary cytokine expression (TNF-α, GM-CSF) decreased, while splenic cytokine (IL-10) increased in binge drunk mice. Increased lung and brain permeability was observed as early as 2 h post alcohol administration in vivo. Trans-epithelial electrical resistance (TEER) was significantly decreased, while intracellular invasion of non-phagocytic cells increased with 0.2% v/v alcohol exposure in vitro.
Conclusions: Our results indicate that a single binge alcohol dose suppressed innate immune functions and increased the ability of less virulent Burkholderia strains to disseminate through increased barrier permeability and intracellular invasion of non-phagocytic cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Antony VB, Godbey SW, Hott JW, Sherry QF. (1993) Alcohol-Induced Inhibition of Alveolar Macrophage Oxidant Release in Vivo and in Vitro. Alcohol Clin Exp Res. 17(2):389–93. - PubMed
-
- Boé DM, Nelson S, Zhang P, Bagby GJ. (2001) Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis. 184(9):1134–42. 10.1086/323661 - DOI - PubMed
-
- Currie BJ. (2015) Melioidosis: Evolving Concepts in Epidemiology, Pathogenesis, and Treatment. Semin Respir Crit Care Med. 36:111–25. 10.1055/s-0034-1398389 - DOI - PubMed
-
- Wiersinga WJ, Currie BJ, Peacock SJ. (2012) Melioidosis. N Engl J Med 367(11):1035–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22970946 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

