Matrix stiffness regulates arteriovenous differentiation of endothelial progenitor cells during vasculogenesis in nude mice
- PMID: 30485569
- PMCID: PMC6495479
- DOI: 10.1111/cpr.12557
Matrix stiffness regulates arteriovenous differentiation of endothelial progenitor cells during vasculogenesis in nude mice
Abstract
Objectives: The aim of the study was to investigate the effect of matrix stiffness on arteriovenous differentiation of endothelial progenitor cells (EPCs) during vasculogenesis in nude mice.
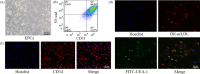
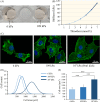
Materials and methods: Dextran hydrogels of differing stiffnesses were first prepared by controlling the crosslinking reaction to generate different thioether bonds. Hydrogels with stiffnesses matching those of the arterial extracellular matrix and venous extracellular matrix were separately combined with mouse bone marrow-derived EPCs and subcutaneously implanted on either side of the backs of nude mice. After 14 days, artery-specific marker Efnb2 and vein-specific marker Ephb4 in the neovasculature were detected to determine the effect of matrix stiffness on the arteriovenous differentiation of EPCs in vivo.
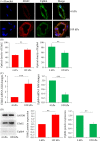
Results: Fourteen days after the implantation of the EPC-loaded dextran hydrogels, new blood vessels were observed in both types of hydrogels. We further verified that matrix stiffness regulated the arteriovenous differentiation of EPCs during vasculogenesis via the Ras/Mek pathway.
Conclusions: Matrix stiffness regulates the arteriovenous differentiation of EPCs during vasculogenesis in nude mice through the Ras/Mek pathway.
Keywords: Ras/Mek pathway; arteriovenous development; matrix characteristics; tissue engineering; vasculogenesis.
© 2018 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 2017;357(6353):eaal2379. - PubMed
-
- Bompais H, Chagraoui J, Canron X, et al. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood. 2004;103(7):2577. - PubMed
-
- Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221‐228. - PubMed
-
- Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288‐293. - PubMed
-
- Obi S, Yamamoto K, Shimizu N, et al. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol. 2009;106(1):203‐211. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

