Extravascular fibrinogen in the white matter of Alzheimer's disease and normal aged brains: implications for fibrinogen as a biomarker for Alzheimer's disease
- PMID: 30485582
- PMCID: PMC8028661
- DOI: 10.1111/bpa.12685
Extravascular fibrinogen in the white matter of Alzheimer's disease and normal aged brains: implications for fibrinogen as a biomarker for Alzheimer's disease
Abstract
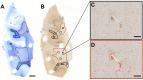
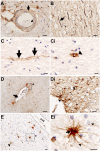
The blood-brain barrier (BBB) regulates cerebrovascular permeability and leakage of blood-derived fibrinogen. Dysfunction of the BBB has been associated with cerebral arteriolosclerosis small vessel disease (SVD) and white matter lesions (WML). Furthermore, BBB dysfunction is associated with the pathogenesis of Alzheimer's disease (AD) with the presence of CSF plasma proteins suggested to be a potential biomarker of AD. We aimed to determine if extravascular fibrinogen in the white matter was associated with the development of AD hallmark pathologies, i.e., hyperphosphorylated tau (HPτ) and amyloid-β (Aβ), as well as SVD, cerebral amyloid angiopathy (CAA) and measures of white matter damage. Using human post-mortem brains, parietal tissue from 20 AD and 22 non-demented controls was quantitatively assessed for HPτ, Aβ, white matter damage severity, axonal density, demyelination and the burden of extravascular fibrinogen in both WML and normal appearing white matter (NAWM). SVD severity was determined by calculating sclerotic indices. WML- and NAWM fibrinogen burden was not significantly different between AD and controls nor was it associated with the burden of HPτ or Aβ pathology, or any measures of white matter damage. Increasing severity of SVD was associated with and a predictor of both higher WML- and NAWM fibrinogen burden (all P < 0.05) in controls only. In cases with minimal SVD NAWM fibrinogen burden was significantly higher in the AD cases (P < 0.05). BBB dysfunction was present in both non-demented and AD brains and was not associated with the burden of AD-associated cortical pathologies. BBB dysfunction was strongly associated with SVD but only in the non-demented controls. In cases with minimal SVD, BBB dysfunction was significantly worse in AD cases possibly indicating the influence of CAA. In conclusion, extravascular fibrinogen is not associated with AD hallmark pathologies but indicates SVD, suggesting that the presence of fibrinogen in the CSF is not a surrogate marker for AD pathology.
Keywords: Alzheimer’s disease; biomarker; blood-brain barrier; cerebral small vessel disease; fibrinogen; white matter hyperintensities; white matter lesion.
© 2018 International Society of Neuropathology.
Figures

Similar articles
-
Frontal white matter lesions in Alzheimer's disease are associated with both small vessel disease and AD-associated cortical pathology.Acta Neuropathol. 2021 Dec;142(6):937-950. doi: 10.1007/s00401-021-02376-2. Epub 2021 Oct 4. Acta Neuropathol. 2021. PMID: 34608542 Free PMC article.
-
Parietal white matter lesions in Alzheimer's disease are associated with cortical neurodegenerative pathology, but not with small vessel disease.Acta Neuropathol. 2017 Sep;134(3):459-473. doi: 10.1007/s00401-017-1738-2. Epub 2017 Jun 21. Acta Neuropathol. 2017. PMID: 28638989 Free PMC article.
-
Cortical tau load is associated with white matter hyperintensities.Acta Neuropathol Commun. 2015 Sep 30;3:60. doi: 10.1186/s40478-015-0240-0. Acta Neuropathol Commun. 2015. PMID: 26419828 Free PMC article.
-
Vascular pathology in the aged human brain.Acta Neuropathol. 2010 Mar;119(3):277-90. doi: 10.1007/s00401-010-0652-7. Epub 2010 Feb 14. Acta Neuropathol. 2010. PMID: 20155424 Free PMC article. Review.
-
Cerebral Small Vessel Disease in Sporadic and Familial Alzheimer Disease.Am J Pathol. 2021 Nov;191(11):1888-1905. doi: 10.1016/j.ajpath.2021.07.004. Epub 2021 Jul 28. Am J Pathol. 2021. PMID: 34331941 Free PMC article. Review.
Cited by
-
Blood-Brain Barrier Dysfunction in Normal Aging and Neurodegeneration: Mechanisms, Impact, and Treatments.Stroke. 2023 Mar;54(3):661-672. doi: 10.1161/STROKEAHA.122.040578. Epub 2023 Feb 27. Stroke. 2023. PMID: 36848419 Free PMC article. Review.
-
The FDP/FIB Ratio and Blood FDP Level May Be Related to Seizures After Fever in Young Children.Front Pediatr. 2020 Jul 31;8:439. doi: 10.3389/fped.2020.00439. eCollection 2020. Front Pediatr. 2020. PMID: 32850552 Free PMC article.
-
Restoring brain barriers: an innovative approach for treating neurological disorders.Fluids Barriers CNS. 2025 Jul 10;22(1):72. doi: 10.1186/s12987-025-00688-z. Fluids Barriers CNS. 2025. PMID: 40640916 Free PMC article. Review.
-
The cognitive dysfunction related to Alzheimer disease or cerebral small vessel disease: What's the differences.Medicine (Baltimore). 2021 Aug 27;100(34):e26967. doi: 10.1097/MD.0000000000026967. Medicine (Baltimore). 2021. PMID: 34449462 Free PMC article.
-
Vascular Dysfunction in Alzheimer's Disease: Alterations in the Plasma Contact and Fibrinolytic Systems.Int J Mol Sci. 2023 Apr 11;24(8):7046. doi: 10.3390/ijms24087046. Int J Mol Sci. 2023. PMID: 37108211 Free PMC article. Review.
References
-
- Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte‐endothelial interactions at the blood‐brain barrier. Nat Rev Neurosci 7:41–53. - PubMed
-
- Alafuzoff I, Adolfsson R, Grundke‐Iqbal I, Winblad B (1987) Blood‐brain barrier in Alzheimer dementia and in non‐demented elderly. An immunocytochemical study. Acta Neuropathol 73:160–166. - PubMed
-
- Attems J, Jellinger KA (2004) Only cerebral capillary amyloid angiopathy correlates with Alzheimer pathology–a pilot study. Acta Neuropathol 107:83–90. - PubMed
-
- Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH et al (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24:197–211. - PubMed

