Cellular Metabolism in Lung Health and Disease
- PMID: 30485759
- PMCID: PMC6853603
- DOI: 10.1146/annurev-physiol-020518-114640
Cellular Metabolism in Lung Health and Disease
Abstract
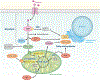
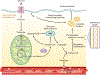
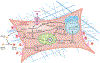
The lung is often overlooked as a metabolically active organ, yet biochemical studies have long demonstrated that glucose utilization surpasses that of many other organs, including the heart, kidney, and brain. For most cells in the lung, energy consumption is relegated to performing common cellular tasks, like mRNA transcription and protein translation. However, certain lung cell populations engage in more specialized types of energy-consuming behaviors, such as the beating of cilia or the production of surfactant. While many extrapulmonary diseases are now linked to abnormalities in cellular metabolism, the pulmonary community has only recently embraced the concept of metabolic dysfunction as a driver of respiratory pathology. Herein, we provide an overview of the major metabolic pathways in the lung and discuss how cells sense and adapt to low-energy states. Moreover, we review some of the emerging evidence that links alterations in cellular metabolism to the pathobiology of several common respiratory diseases.
Keywords: cellular metabolism; energy; glycolysis; lung; mitochondria; respiratory disease.
Figures

References
-
- Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, et al. 2001. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 128:5181–88 - PubMed
-
- Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. 2003. Side population cells and Bcrp1 expression in lung. Am. J. Physiol. Lung Cell. Mol. Physiol 285:L97–104 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

