Branched Chain Amino Acids
- PMID: 30485760
- PMCID: PMC6536377
- DOI: 10.1146/annurev-physiol-020518-114455
Branched Chain Amino Acids
Abstract
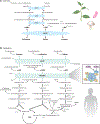
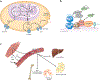
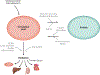
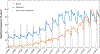
Branched chain amino acids (BCAAs) are building blocks for all life-forms. We review here the fundamentals of BCAA metabolism in mammalian physiology. Decades of studies have elicited a deep understanding of biochemical reactions involved in BCAA catabolism. In addition, BCAAs and various catabolic products act as signaling molecules, activating programs ranging from protein synthesis to insulin secretion. How these processes are integrated at an organismal level is less clear. Inborn errors of metabolism highlight the importance of organismal regulation of BCAA physiology. More recently, subtle alterations of BCAA metabolism have been suggested to contribute to numerous prevalent diseases, including diabetes, cancer, and heart failure. Understanding the mechanisms underlying altered BCAA metabolism and how they contribute to disease pathophysiology will keep researchers busy for the foreseeable future.
Keywords: branched chain amino acids; cancer; catabolism; diabetes; heart disease.
Figures
References
-
- Davis TA, Fiorotto ML, Reeds PJ. 1993. Amino acid compositions of body and milk protein change during the suckling period in rats. J. Nutr 123(5):947–56 - PubMed
-
- Harper A, Block K, Cree T. 1983. Branched-chain amino acids: nutritional and metabolic interrelationships In Proceedings of the Fourth Symposium on Protein Metabolism and Nutrition, ed. Pion R, Arnal M, Bonin D, pp. 159–81. Paris: INRA
-
- Chou PY, Fasman GD. 1973. Structural and functional role of leucine residues in proteins. J. Mol. Biol 74(3):263–81 - PubMed
-
- Dill KA. 1990. Dominant forces in protein folding. Biochemistry 29(31):7133–55 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

