Mitochondrial Iron in Human Health and Disease
- PMID: 30485761
- PMCID: PMC6641538
- DOI: 10.1146/annurev-physiol-020518-114742
Mitochondrial Iron in Human Health and Disease
Abstract
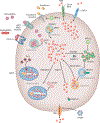
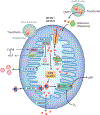
Mitochondria are an iconic distinguishing feature of eukaryotic cells. Mitochondria encompass an active organellar network that fuses, divides, and directs a myriad of vital biological functions, including energy metabolism, cell death regulation, and innate immune signaling in different tissues. Another crucial and often underappreciated function of these dynamic organelles is their central role in the metabolism of the most abundant and biologically versatile transition metals in mammalian cells, iron. In recent years, cellular and animal models of mitochondrial iron dysfunction have provided vital information in identifying new proteins that have elucidated the pathways involved in mitochondrial homeostasis and iron metabolism. Specific signatures of mitochondrial iron dysregulation that are associated with disease pathogenesis and/or progression are becoming increasingly important. Understanding the molecular mechanisms regulating mitochondrial iron pathways will help better define the role of this important metal in mitochondrial function and in human health and disease.
Keywords: iron; metabolism; mitochondria.
Conflict of interest statement
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Figures
Similar articles
-
Mitochondria in lung disease.J Clin Invest. 2016 Mar 1;126(3):809-20. doi: 10.1172/JCI81113. Epub 2016 Mar 1. J Clin Invest. 2016. PMID: 26928034 Free PMC article. Review.
-
Mitochondrial mayhem: the mitochondrion as a modulator of iron metabolism and its role in disease.Antioxid Redox Signal. 2011 Dec 15;15(12):3003-19. doi: 10.1089/ars.2011.3921. Epub 2011 May 5. Antioxid Redox Signal. 2011. PMID: 21545274 Review.
-
Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease.Biochim Biophys Acta. 2015 May;1853(5):1130-44. doi: 10.1016/j.bbamcr.2015.01.021. Epub 2015 Feb 4. Biochim Biophys Acta. 2015. PMID: 25661197 Review.
-
The interplay between mitochondrial protein and iron homeostasis and its possible role in ageing.Exp Gerontol. 2014 Aug;56:123-34. doi: 10.1016/j.exger.2013.12.015. Epub 2014 Jan 4. Exp Gerontol. 2014. PMID: 24394155 Review.
-
Mitochondrial involvement in genetically determined transition metal toxicity I. Iron toxicity.Chem Biol Interact. 2006 Oct 27;163(1-2):68-76. doi: 10.1016/j.cbi.2006.05.007. Epub 2006 May 17. Chem Biol Interact. 2006. PMID: 16797509 Review.
Cited by
-
Noncoding RNAs regulating ferroptosis in cardiovascular diseases: novel roles and therapeutic strategies.Mol Cell Biochem. 2024 Nov;479(11):2827-2841. doi: 10.1007/s11010-023-04895-w. Epub 2023 Dec 8. Mol Cell Biochem. 2024. PMID: 38064139 Free PMC article. Review.
-
Iron Metabolism in Obesity and Metabolic Syndrome.Int J Mol Sci. 2020 Aug 1;21(15):5529. doi: 10.3390/ijms21155529. Int J Mol Sci. 2020. PMID: 32752277 Free PMC article. Review.
-
Clinical and Molecular Correlates of Abnormal Changes in the Cerebellum and Globus Pallidus in Fragile X Premutation.Front Neurol. 2022 Feb 8;13:797649. doi: 10.3389/fneur.2022.797649. eCollection 2022. Front Neurol. 2022. PMID: 35211082 Free PMC article.
-
Targeting ferroptosis for the treatment of female reproductive system disorders.J Mol Med (Berl). 2025 Apr;103(4):381-402. doi: 10.1007/s00109-025-02528-x. Epub 2025 Mar 18. J Mol Med (Berl). 2025. PMID: 40100417 Review.
-
Targeting Ferroptosis: Small-molecule Inducers as Novel Anticancer Agents.Anticancer Agents Med Chem. 2025;25(8):517-532. doi: 10.2174/0118715206342278241008081126. Anticancer Agents Med Chem. 2025. PMID: 39411969 Review.
References
-
- Rauen U, Springer A, Weisheit D, Petrat F, Korth HG, et al. 2007. Assessment of chelatable mitochondrial iron by using mitochondrion-selective fluorescent iron indicators with different iron-binding affinities. ChemBioChem 8:341–52 - PubMed
-
- Chevion M 1988. A site-specific mechanism for free radical induced biological damage: the essential role of redox-active transition metals. Free Radic. Biol. Med 5:27–37 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

