Microtubule-Based Control of Motor-Clutch System Mechanics in Glioma Cell Migration
- PMID: 30485822
- PMCID: PMC6345402
- DOI: 10.1016/j.celrep.2018.10.101
Microtubule-Based Control of Motor-Clutch System Mechanics in Glioma Cell Migration
Abstract
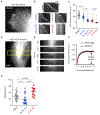
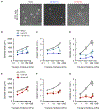
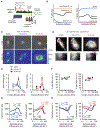
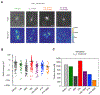
Microtubule-targeting agents (MTAs) are widely used chemotherapy drugs capable of disrupting microtubule-dependent cellular functions, such as division and migration. We show that two clinically approved MTAs, paclitaxel and vinblastine, each suppress stiffness-sensitive migration and polarization characteristic of human glioma cells on compliant hydrogels. MTAs influence microtubule dynamics and cell traction forces by nearly opposite mechanisms, the latter of which can be explained by a combination of changes in myosin motor and adhesion clutch number. Our results support a microtubule-dependent signaling-based model for controlling traction forces through a motor-clutch mechanism, rather than microtubules directly relieving tension within F-actin and adhesions. Computational simulations of cell migration suggest that increasing protrusion number also impairs stiffness-sensitive migration, consistent with experimental MTA effects. These results provide a theoretical basis for the role of microtubules and mechanisms of MTAs in controlling cell migration.
Keywords: actin; cell migration; computational modeling; cytoskeletal crosstalk; mechanotransduction; microtubule; microtubule-targeting agent; paclitaxel; receptor tyrosine kinase; vinblastine.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures


References
-
- Belotti D, Rieppi M, Nicoletti MI, Casazza AM, Fojo T, Taraboletti G, and Giavazzi R (1996). Paclitaxel (Taxol(R)) inhibits motility of paclitaxel-resistant human ovarian carcinoma cells. Clin. Cancer Res 2, 1725–1730. - PubMed
-
- Bergès R, Tchoghandjian A, Honoré S, Estève M-A, Figarella-Branger D, Bachmann F, Lane HA, and Braguer D (2016). The novel tubulin-binding checkpoint activator BAL101553 inhibits EB1-dependent migration and invasion and promotes differentiation of glioblastoma stem-like cells. Mol. Cancer Ther 75, 2740–2749. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

