Clogging the Ubiquitin-Proteasome Machinery with Marine Natural Products: Last Decade Update
- PMID: 30486251
- PMCID: PMC6316072
- DOI: 10.3390/md16120467
Clogging the Ubiquitin-Proteasome Machinery with Marine Natural Products: Last Decade Update
Abstract
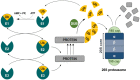
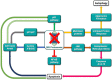
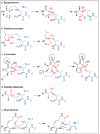
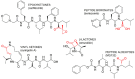
The ubiquitin-proteasome pathway (UPP) is the central protein degradation system in eukaryotic cells, playing a key role in homeostasis maintenance, through proteolysis of regulatory and misfolded (potentially harmful) proteins. As cancer cells produce proteins inducing cell proliferation and inhibiting cell death pathways, UPP inhibition has been exploited as an anticancer strategy to shift the balance between protein synthesis and degradation towards cell death. Over the last few years, marine invertebrates and microorganisms have shown to be an unexhaustive factory of secondary metabolites targeting the UPP. These chemically intriguing compounds can inspire clinical development of novel antitumor drugs to cope with the incessant outbreak of side effects and resistance mechanisms induced by currently approved proteasome inhibitors (e.g., bortezomib). In this review, we report about (a) the role of the UPP in anticancer therapy, (b) chemical and biological properties of UPP inhibitors from marine sources discovered in the last decade, (c) high-throughput screening techniques for mining natural UPP inhibitors in organic extracts. Moreover, we will tell about the fascinating story of salinosporamide A, the first marine natural product to access clinical trials as a proteasome inhibitor for cancer treatment.
Keywords: cancer; high-throughput screening; lead compounds; marine; natural products; proteasome; salinosporamide; secondary metabolites; ubiquitin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Discovery of Natural Proteasome Inhibitors as Novel Anticancer Therapeutics: Current Status and Perspectives.Curr Protein Pept Sci. 2018 Feb 13;19(4):358-367. doi: 10.2174/1389203718666170111121856. Curr Protein Pept Sci. 2018. PMID: 28079010 Review.
-
Search for Inhibitors of the Ubiquitin-Proteasome System from Natural Sources for Cancer Therapy.Chem Pharm Bull (Tokyo). 2016;64(2):112-8. doi: 10.1248/cpb.c15-00768. Chem Pharm Bull (Tokyo). 2016. PMID: 26833439 Review.
-
Cell-based screening of extracts of natural sources to search for inhibitors of the ubiquitin-proteasome system and identification of proteasome inhibitors from the fungus Remotididymella sp.Bioorg Med Chem Lett. 2022 Mar 1;59:128566. doi: 10.1016/j.bmcl.2022.128566. Epub 2022 Jan 19. Bioorg Med Chem Lett. 2022. PMID: 35063633
-
Novel copper complexes as potential proteasome inhibitors for cancer treatment (Review).Mol Med Rep. 2017 Jan;15(1):3-11. doi: 10.3892/mmr.2016.6022. Epub 2016 Dec 9. Mol Med Rep. 2017. PMID: 27959411 Review.
-
A High-Content Screening Assay for the Discovery of Novel Proteasome Inhibitors from Formosan Soft Corals.Mar Drugs. 2018 Oct 21;16(10):395. doi: 10.3390/md16100395. Mar Drugs. 2018. PMID: 30347865 Free PMC article.
Cited by
-
The Molecular Basis of Ubiquitin-Conjugating Enzymes (E2s) as a Potential Target for Cancer Therapy.Int J Mol Sci. 2021 Mar 26;22(7):3440. doi: 10.3390/ijms22073440. Int J Mol Sci. 2021. PMID: 33810518 Free PMC article. Review.
-
Enzyme Inhibitors from Gorgonians and Soft Corals.Mar Drugs. 2023 Jan 31;21(2):104. doi: 10.3390/md21020104. Mar Drugs. 2023. PMID: 36827145 Free PMC article. Review.
-
A Collection of Bioactive Nitrogen-Containing Molecules from the Marine Sponge Acanthostrongylophora ingens.Mar Drugs. 2019 Aug 15;17(8):472. doi: 10.3390/md17080472. Mar Drugs. 2019. PMID: 31443260 Free PMC article.
-
Dichloroacetate (DCA) and Cancer: An Overview towards Clinical Applications.Oxid Med Cell Longev. 2019 Nov 14;2019:8201079. doi: 10.1155/2019/8201079. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31827705 Free PMC article. Review.
-
Toxicogenomics of the Freshwater Oligochaete, Tubifex tubifex (Annelida, Clitellata), in Acute Water-Only Exposure to Arsenic.Int J Mol Sci. 2024 Mar 16;25(6):3382. doi: 10.3390/ijms25063382. Int J Mol Sci. 2024. PMID: 38542353 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

