Regenerative Models for the Integration and Regeneration of Head Skeletal Tissues
- PMID: 30486286
- PMCID: PMC6321600
- DOI: 10.3390/ijms19123752
Regenerative Models for the Integration and Regeneration of Head Skeletal Tissues
Abstract
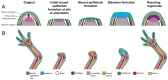
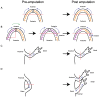
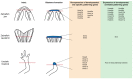
Disease of, or trauma to, the human jaw account for thousands of reconstructive surgeries performed every year. One of the most popular and successful treatment options in this context involves the transplantation of bone tissue from a different anatomical region into the affected jaw. Although, this method has been largely successful, the integration of the new bone into the existing bone is often imperfect, and the integration of the host soft tissues with the transplanted bone can be inconsistent, resulting in impaired function. Unlike humans, several vertebrate species, including fish and amphibians, demonstrate remarkable regenerative capabilities in response to jaw injury. Therefore, with the objective of identifying biological targets to promote and engineer improved outcomes in the context of jaw reconstructive surgery, we explore, compare and contrast the natural mechanisms of endogenous jaw and limb repair and regeneration in regenerative model organisms. We focus on the role of different cell types as they contribute to the regenerating structure; how mature cells acquire plasticity in vivo; the role of positional information in pattern formation and tissue integration, and limitations to endogenous regenerative and repair mechanisms.
Keywords: axolotl; jaw regeneration; limb regeneration; positional information; regenerative medicine; transplantation; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- McCarthy J.G. The role of distraction osteogenesis in the reconstruction of the mandible in unilateral craniofacial microsomia. Clin. Plast. Surg. 1994;21:625–631. - PubMed
-
- Shwyrkow M.B., Shamsudinov A.K. Methods of simultaneous treatment of the mandible defects and the adjacent soft tissues. Acta Chir. Plast. 1989;31:226–235. - PubMed
-
- Komisar A. Mandibular Reconstruction. Thieme; Stuttgart, Germany: 1997.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

