Applications of Gold Nanoparticles in Non-Optical Biosensors
- PMID: 30486293
- PMCID: PMC6315477
- DOI: 10.3390/nano8120977
Applications of Gold Nanoparticles in Non-Optical Biosensors
Abstract
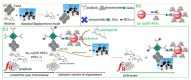
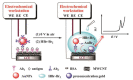
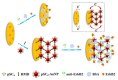
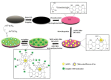
Due to their unique properties, such as good biocompatibility, excellent conductivity, effective catalysis, high density, and high surface-to-volume ratio, gold nanoparticles (AuNPs) are widely used in the field of bioassay. Mainly, AuNPs used in optical biosensors have been described in some reviews. In this review, we highlight recent advances in AuNP-based non-optical bioassays, including piezoelectric biosensor, electrochemical biosensor, and inductively coupled plasma mass spectrometry (ICP-MS) bio-detection. Some representative examples are presented to illustrate the effect of AuNPs in non-optical bioassay and the mechanisms of AuNPs in improving detection performances are described. Finally, the review summarizes the future prospects of AuNPs in non-optical biosensors.
Keywords: ICP-MS; biosensor; electrochemical; gold nanoparticles; piezoelectric.
Conflict of interest statement
The authors declare no conflict of interest. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Figures

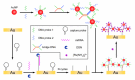
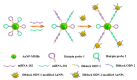
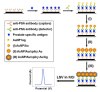

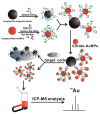
References
-
- Gole A., Dash C., Ramakrishnan V., Sainkar S.R., Mandale A.B., Rao M., Sastry M. Pepsin−gold colloid conjugates: Preparation, characterization, and enzymatic activity. Langmuir. 2001;17:1674–1679. doi: 10.1021/la001164w. - DOI
Publication types
Grants and funding
- 201711535035/Research and Innovative Experiment Program for College Students in Hunan Province
- CNXY2017009/Open Fund of Guangdong Provincial Characteristic Key Discipline of Material Science
- 2016GCZX008/the Key Project of Department of Education of Guangdong Province
- 20172010018/the Project of Engineering Research Center of Foshan
LinkOut - more resources
Full Text Sources

