Involvement of Carnosic Acid in the Phytotoxicity of Rosmarinus officinalis Leaves
- PMID: 30486296
- PMCID: PMC6316382
- DOI: 10.3390/toxins10120498
Involvement of Carnosic Acid in the Phytotoxicity of Rosmarinus officinalis Leaves
Abstract
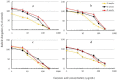
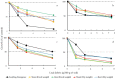
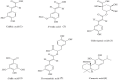
Weeds are rapidly developing resistance to synthetic herbicides, and this can pose a threat to the ecosystem. Exploring allelopathic species as an alternative weed control measure can help minimize the ecological threat posed by herbicide-resistant weeds. In this study, we aimed to evaluate the contribution of some polyphenols to the allelopathy of rosemary (Rosmarinus officinalis L.). The phytotoxic effects of rosemary (leaves, roots, inflorescences, and stems) crude extracts were tested on lettuce (Lactuca sativa L.). Soils incorporated with dried rosemary leaves were also tested on test plants. Reversed-phase high-performance liquid chromatography (HPLC) analysis was used to determine the content of some polyphenols (caffeic, ferulic, gallic, rosmarinic, carnosic, and chlorogenic acids) in rosemary. The specific activity and total activity of crude extracts and individual compounds were evaluated using lettuce. The crude extract of rosemary leaves showed the highest growth inhibitory effect among the rosemary organs tested. Soil amended with rosemary leaf debris reduced the dry matter and seed emergence of lettuce. Carnosic acid was the main compound detected in rosemary leaves and had a high specific activity when tested on lettuce. During the seed germination period, there was observed filter paper coloration among the test plants treated with carnosic acid (250 μg/mL). The high concentration and strong inhibitory effect of carnosic acid could explain the inhibitory activity of the rosemary leaf extract. Hence, we conclude based on the total activity estimation that carnosic acid among the other tested compounds is the major allelochemical in rosemary leaves.
Keywords: Rosmarinus officinalis; allelopathy; carnosic acid; inhibitory; phytotoxicity; specific activity; total activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Willis R.J. The History of Allelopathy. Springer; Dordrecht, The Netherlands: 2007. What Is Allelopathy? pp. 1–13.
-
- Inderjit, Weston L., Duke S. Challenges, Achievements and Opportunities in Allelopathy Research. J. Plant Interact. 2005;1:69–81. doi: 10.1080/17429140600622535. - DOI
-
- Xuan T.D., Shinkichi T., Khanh T.D., Chung I.M. Biological Control of Weeds and Plant Pathogens in Paddy Rice by Exploiting Plant Allelopathy: An Overview. Crop Prot. 2005;24:197–206. doi: 10.1016/j.cropro.2004.08.004. - DOI
-
- Fujii Y., Shibuya T., Tamaki Y. Allelopathy of Velvetbean: Determination and Identification of L-DOPA as a Candidate of Allelopathic Substances. Japan Agric. Res. Q. 1992;25:238–247.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

