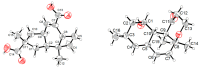Chemical Constituents of the Marine-Derived Fungus Aspergillus sp. SCS-KFD66
- PMID: 30486303
- PMCID: PMC6316597
- DOI: 10.3390/md16120468
Chemical Constituents of the Marine-Derived Fungus Aspergillus sp. SCS-KFD66
Abstract
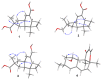
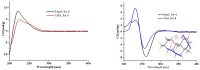
Five new compounds named asperpenes A-C (1⁻3), 12,13-dedihydroversiol (4), and methyl 6-oxo-3,6-dihydro-2H-pyran-4-carboxylate (5), along with 10 known compounds (6⁻15), were isolated from the fermentation broth of Aspergillus sp. SCS-KFD66 associated with a bivalve mollusk, Sanguinolaria chinensis, collected from Haikou Bay, China. The structures of the compounds, including the absolute configurations of their stereogenic carbons, were unambiguously determined by spectroscopic data, single-crystal X-ray diffraction analysis, and electronic circular dichroism (ECD) spectral analysis, along with quantum ECD calculations. The growth inhibitory activity of the compounds against four pathogenic bacterial (Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 6538, Listeria monocytogenes ATCC 1911, and Bacillus subtilis ATCC 6633), their enzyme inhibitory activities against acetylcholinesterase and α-glucosidase, and their DPPH radical scavenging activity were evaluated.
Keywords: Aspergillus sp.; antibacterial activity; marine-derived fungus; secondary metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Asperpenes D and E from the fungus Aspergillus sp. SCS-KFD66 isolated from a bivalve mollusk, Sanguinolaria chinensis.J Asian Nat Prod Res. 2021 Feb;23(2):117-122. doi: 10.1080/10286020.2019.1709450. Epub 2020 Jan 24. J Asian Nat Prod Res. 2021. PMID: 31979983
-
Diketopiperazine and Diphenylether Derivatives from Marine Algae-Derived Aspergillus versicolor OUCMDZ-2738 by Epigenetic Activation.Mar Drugs. 2018 Dec 22;17(1):6. doi: 10.3390/md17010006. Mar Drugs. 2018. PMID: 30583513 Free PMC article.
-
Asperversins A and B, Two Novel Meroterpenoids with an Unusual 5/6/6/6 Ring from the Marine-Derived Fungus Aspergillus versicolor.Mar Drugs. 2018 May 23;16(6):177. doi: 10.3390/md16060177. Mar Drugs. 2018. PMID: 29882867 Free PMC article.
-
Antibacterial properties of natural products from marine fungi reported between 2012 and 2023: a review.Arch Pharm Res. 2024 Jun;47(6):505-537. doi: 10.1007/s12272-024-01500-6. Epub 2024 Jun 8. Arch Pharm Res. 2024. PMID: 38850495 Review.
-
Marine Aspergillus: A Treasure Trove of Antimicrobial Compounds.Mar Drugs. 2023 Apr 28;21(5):277. doi: 10.3390/md21050277. Mar Drugs. 2023. PMID: 37233471 Free PMC article. Review.
Cited by
-
Cytotoxic Polyketides Isolated from the Deep-Sea-Derived Fungus Penicillium chrysogenum MCCC 3A00292.Mar Drugs. 2019 Dec 5;17(12):686. doi: 10.3390/md17120686. Mar Drugs. 2019. PMID: 31817515 Free PMC article.
-
Divergolides T⁻W with Apoptosis-Inducing Activity from the Mangrove-Derived Actinomycete Streptomyces sp. KFD18.Mar Drugs. 2019 Apr 11;17(4):219. doi: 10.3390/md17040219. Mar Drugs. 2019. PMID: 30978939 Free PMC article.
-
Research Advances of Bioactive Sesquiterpenoids Isolated from Marine-Derived Aspergillus sp.Molecules. 2022 Oct 30;27(21):7376. doi: 10.3390/molecules27217376. Molecules. 2022. PMID: 36364202 Free PMC article. Review.
-
Recent updates on the bioactive compounds of the marine-derived genus Aspergillus.RSC Adv. 2021 May 11;11(28):17116-17150. doi: 10.1039/d1ra01359a. eCollection 2021 May 6. RSC Adv. 2021. PMID: 35479707 Free PMC article. Review.
-
Significance of research on natural products from marine-derived Aspergillus species as a source against pathogenic bacteria.Front Microbiol. 2024 Sep 19;15:1464135. doi: 10.3389/fmicb.2024.1464135. eCollection 2024. Front Microbiol. 2024. PMID: 39364162 Free PMC article. Review.
References
-
- Hamed I., Ozogul F., Ozogul Y., Regenstein J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015;14:446–465. doi: 10.1111/1541-4337.12136. - DOI
-
- Kong F.D., Ma Q.Y., Huang S.Z., Wang P., Wang J.F., Zhou L.M., Yuan J.Z., Dai H.F., Zhao Y.X. Chrodrimanins K-N and related meroterpenoids from the fungus Penicillium sp. SCS-KFD09 isolated from a marine worm, Sipunculus nudus. J. Nat. Prod. 2017;80:1039–1047. doi: 10.1021/acs.jnatprod.6b01061. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases