Expression, Localization, and Activity of the Aryl Hydrocarbon Receptor in the Human Placenta
- PMID: 30486367
- PMCID: PMC6321474
- DOI: 10.3390/ijms19123762
Expression, Localization, and Activity of the Aryl Hydrocarbon Receptor in the Human Placenta
Abstract
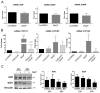
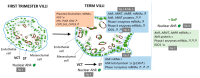
The human placenta is an organ between the blood of the mother and the fetus, which is essential for fetal development. It also plays a role as a selective barrier against environmental pollutants that may bypass epithelial barriers and reach the placenta, with implications for the outcome of pregnancy. The aryl hydrocarbon receptor (AhR) is one of the most important environmental-sensor transcription factors and mediates the metabolism of a wide variety of xenobiotics. Nevertheless, the identification of dietary and endogenous ligands of AhR suggest that it may also fulfil physiological functions with which pollutants may interfere. Placental AhR expression and activity is largely unknown. We established the cartography of AhR expression at transcript and protein levels, its cellular distribution, and its transcriptional activity toward the expression of its main target genes. We studied the profile of AhR expression and activity during different pregnancy periods, during trophoblasts differentiation in vitro, and in a trophoblast cell line. Using diverse methods, such as cell fractionation and immunofluorescence microscopy, we found a constitutive nuclear localization of AhR in every placental model, in the absence of any voluntarily-added exogenous activator. Our data suggest an intrinsic activation of AhR due to the presence of endogenous placental ligands.
Keywords: ARNT; AhR; CYP1A1; CYP1B1; benzo-a-pyrene; ontogeny; placenta; trophoblast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
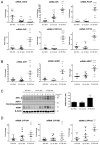
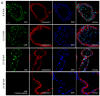


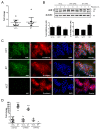


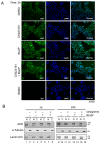
References
-
- Juricek L., Bui L.C., Busi F., Pierre S., Guyot E., Lamouri A., Dupret J.M., Barouki R., Coumoul X., Rodrigues-Lima F. Activation of the aryl hydrocarbon receptor by carcinogenic aromatic amines and modulatory effects of their N-acetylated metabolites. Arch. Toxicol. 2015;89:2403–2412. doi: 10.1007/s00204-014-1367-7. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

