Blood Outgrowth and Proliferation of Endothelial Colony Forming Cells are Related to Markers of Disease Severity in Patients with Pulmonary Arterial Hypertension
- PMID: 30486375
- PMCID: PMC6321271
- DOI: 10.3390/ijms19123763
Blood Outgrowth and Proliferation of Endothelial Colony Forming Cells are Related to Markers of Disease Severity in Patients with Pulmonary Arterial Hypertension
Abstract
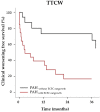
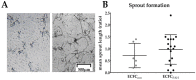
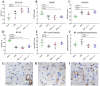
In pulmonary arterial hypertension (PAH), lung-angioproliferation leads to increased pulmonary vascular resistance, while simultaneous myocardial microvessel loss contributes to right ventricular (RV) failure. Endothelial colony forming cells (ECFC) are highly proliferative, angiogenic cells that may contribute to either pulmonary vascular obstruction or to RV microvascular adaptation. We hypothesize ECFC phenotypes (outgrowth, proliferation, tube formation) are related to markers of disease severity in a prospective cohort-study of 33 PAH and 30 healthy subjects. ECFC were transplanted in pulmonary trunk banded rats with RV failure. The presence of ECFC outgrowth in PAH patients was associated with low RV ejection fraction, low central venous saturation and a shorter time to clinical worsening (5.4 months (0.6⁻29.2) vs. 36.5 months (7.4⁻63.4), p = 0.032). Functionally, PAH ECFC had higher proliferative rates compared to control in vitro, although inter-patient variability was high. ECFC proliferation was inversely related to RV end diastolic volume (R² = 0.39, p = 0.018), but not pulmonary vascular resistance. Tube formation-ability was similar among donors. Normal and highly proliferative PAH ECFC were transplanted in pulmonary trunk banded rats. While no effect on hemodynamic measurements was observed, RV vascular density was restored. In conclusion, we found that ECFC outgrowth associates with high clinical severity in PAH, suggesting recruitment. Transplantation of highly proliferative ECFC restored myocardial vascular density in pulmonary trunk banded rats, while RV functional improvements were not observed.
Keywords: endothelial colony forming cell; endothelial progenitor cell; pulmonary hypertension; pulmonary vascular disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Vonk-Noordegraaf A., Haddad F.O., Chin K.M., Forfia P.R., Kawut S.M., Lumens J., Naeije R., Newman J., Oudiz R.J., Provencher S., et al. Right Heart Adaptation to Pulmonary Arterial-Hypertension: Physiology and Pathobiology. J. Am. Coll. Cardiol. 2013;62:D22–D33. doi: 10.1016/j.jacc.2013.10.027. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

