How do Nucleotides Adsorb Onto Clays?
- PMID: 30486384
- PMCID: PMC6316844
- DOI: 10.3390/life8040059
How do Nucleotides Adsorb Onto Clays?
Abstract
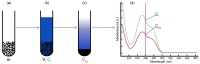
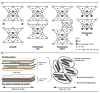
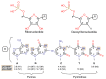
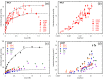
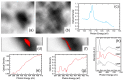
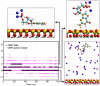
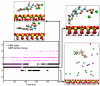
Adsorption of prebiotic building blocks is proposed to have played a role in the emergence of life on Earth. The experimental and theoretical study of this phenomenon should be guided by our knowledge of the geochemistry of the habitable early Earth environments, which could have spanned a large range of settings. Adsorption being an interfacial phenomenon, experiments can be built around the minerals that probably exhibited the largest specific surface areas and were the most abundant, i.e., phyllosilicates. Our current work aims at understanding how nucleotides, the building blocks of RNA and DNA, might have interacted with phyllosilicates under various physico-chemical conditions. We carried out and refined batch adsorption studies to explore parameters such as temperature, pH, salinity, etc. We built a comprehensive, generalized model of the adsorption mechanisms of nucleotides onto phyllosilicate particles, mainly governed by phosphate reactivity. More recently, we used surface chemistry and geochemistry techniques, such as vibrational spectroscopy, low pressure gas adsorption, X-ray microscopy, and theoretical simulations, in order to acquire direct data on the adsorption configurations and localization of nucleotides on mineral surfaces. Although some of these techniques proved to be challenging, questioning our ability to easily detect biosignatures, they confirmed and complemented our pre-established model.
Keywords: adsorption; geochemistry; origins of life; phyllosilicates; surface chemistry.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Hazen R.M., Sverjensky D.A., Azzolini D., Bish D.L., Elmore S.C., Hinnov L., Milliken R.E. Clay mineral evolution. Am. Mineral. 2013;98:2007–2029. doi: 10.2138/am.2013.4425. - DOI
-
- Giese R.F., Van Oss C.J., Yariv S., Cross H. Organo-Clay Complexes and Interactions. Marcel Dekker; New York, NY, USA: 2002. Organophilicity and hydrophobicity of organo-clays; pp. 175–192.
-
- Bernal J.D. The Physical Basis of Life. Proc. Phys. Soc. Sect. B. 1949;62:598–618. doi: 10.1088/0370-1301/62/11/507. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

