Exploiting the Electronic Tuneability of Carboranes as Supports for Frustrated Lewis Pairs
- PMID: 30486434
- PMCID: PMC6321043
- DOI: 10.3390/molecules23123099
Exploiting the Electronic Tuneability of Carboranes as Supports for Frustrated Lewis Pairs
Abstract
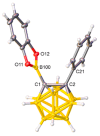
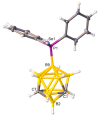
The first example of a carborane with a catecholborolyl substituent, [1-Bcat-2-Ph-closo-1,2-C₂B10H10] (1), has been prepared and characterized and shown to act as the Lewis acid component of an intermolecular frustrated Lewis pair in catalyzing a Michael addition. In combination with B(C₆F₅)₃ the C-carboranylphosphine [1-PPh₂-closo-1,2-C₂B10H11] (IVa) is found to be comparable with PPh₂(C₆F₅) in its ability to catalyze hydrosilylation, whilst the more strongly basic B-carboranylphosphine [9-PPh₂-closo-1,7-C₂B10H11] (V) is less effective and the very weakly basic species [μ-2,2'-PPh-{1-(1'-1',2'-closo-C₂B10H10)-1,2-closo-C₂B10H10}] (IX) is completely ineffective. Base strengths are rank-ordered via measurement of the ¹J 31P-77Se coupling constants of the phosphineselenides [1-SePPh₂-closo-1,2-C₂B10H11] (2), [9-SePPh₂-closo-1,7-C₂B10H11] (3), and [SePPh₂(C₆F₅)] (4).
Keywords: carborane; catalysis; frustrated Lewis pair; phosphine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
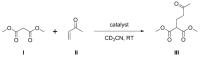




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

