Piecing Together How Peroxiredoxins Maintain Genomic Stability
- PMID: 30486489
- PMCID: PMC6316004
- DOI: 10.3390/antiox7120177
Piecing Together How Peroxiredoxins Maintain Genomic Stability
Abstract
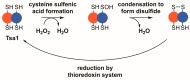
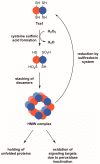
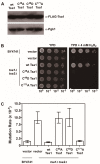
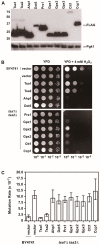
Peroxiredoxins, a highly conserved family of thiol oxidoreductases, play a key role in oxidant detoxification by partnering with the thioredoxin system to protect against oxidative stress. In addition to their peroxidase activity, certain types of peroxiredoxins possess other biochemical activities, including assistance in preventing protein aggregation upon exposure to high levels of oxidants (molecular chaperone activity), and the transduction of redox signals to downstream proteins (redox switch activity). Mice lacking the peroxiredoxin Prdx1 exhibit an increased incidence of tumor formation, whereas baker's yeast (Saccharomyces cerevisiae) lacking the orthologous peroxiredoxin Tsa1 exhibit a mutator phenotype. Collectively, these findings suggest a potential link between peroxiredoxins, control of genomic stability, and cancer etiology. Here, we examine the potential mechanisms through which Tsa1 lowers mutation rates, taking into account its diverse biochemical roles in oxidant defense, protein homeostasis, and redox signaling as well as its interplay with thioredoxin and thioredoxin substrates, including ribonucleotide reductase. More work is needed to clarify the nuanced mechanism(s) through which this highly conserved peroxidase influences genome stability, and to determine if this mechanism is similar across a range of species.
Keywords: genomic instability; mutator; oxidative stress; peroxiredoxin; redox switch; ribonucleotide reductase; sulfiredoxin; thiol peroxidase; thioredoxin.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

