Chemically Induced Cellular Proteolysis: An Emerging Therapeutic Strategy for Undruggable Targets
- PMID: 30486612
- PMCID: PMC6277563
- DOI: 10.14348/molcells.2018.0372
Chemically Induced Cellular Proteolysis: An Emerging Therapeutic Strategy for Undruggable Targets
Abstract
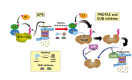
Traditionally, small-molecule or antibody-based therapies against human diseases have been designed to inhibit the enzymatic activity or compete for the ligand binding sites of pathological target proteins. Despite its demonstrated effectiveness, such as in cancer treatment, this approach is often limited by recurring drug resistance. More importantly, not all molecular targets are enzymes or receptors with druggable 'hot spots' that can be directly occupied by active site-directed inhibitors. Recently, a promising new paradigm has been created, in which small-molecule chemicals harness the naturally occurring protein quality control machinery of the ubiquitin-proteasome system to specifically eradicate disease-causing proteins in cells. Such 'chemically induced protein degradation' may provide unprecedented opportunities for targeting proteins that are inherently undruggable, such as structural scaffolds and other non-enzymatic molecules, for therapeutic purposes. This review focuses on surveying recent progress in developing E3-guided proteolysis-targeting chimeras (PROTACs) and small-molecule chemical modulators of deubiquitinating enzymes upstream of or on the proteasome.
Keywords: PROTAC; deubiquitinating enzyme; induced proteolysis; small-molecules; ubiquitin-proteasome system; undruggable target.
Figures
Similar articles
-
Proteasome substrate receptors and their therapeutic potential.Trends Biochem Sci. 2022 Nov;47(11):950-964. doi: 10.1016/j.tibs.2022.06.006. Epub 2022 Jul 9. Trends Biochem Sci. 2022. PMID: 35817651 Free PMC article. Review.
-
PROTAC-DB 2.0: an updated database of PROTACs.Nucleic Acids Res. 2023 Jan 6;51(D1):D1367-D1372. doi: 10.1093/nar/gkac946. Nucleic Acids Res. 2023. PMID: 36300631 Free PMC article.
-
Small-molecule control of intracellular protein levels through modulation of the ubiquitin proteasome system.Angew Chem Int Ed Engl. 2014 Feb 24;53(9):2312-30. doi: 10.1002/anie.201307761. Epub 2014 Jan 23. Angew Chem Int Ed Engl. 2014. PMID: 24459094 Free PMC article. Review.
-
Proteolysis targeting chimeras (PROTACs) are emerging therapeutics for hematologic malignancies.J Hematol Oncol. 2020 Jul 27;13(1):103. doi: 10.1186/s13045-020-00924-z. J Hematol Oncol. 2020. PMID: 32718354 Free PMC article. Review.
-
Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead.Cell Chem Biol. 2018 Jan 18;25(1):78-87.e5. doi: 10.1016/j.chembiol.2017.09.010. Epub 2017 Nov 9. Cell Chem Biol. 2018. PMID: 29129718 Free PMC article.
Cited by
-
An overview of PROTACs: a promising drug discovery paradigm.Mol Biomed. 2022 Dec 20;3(1):46. doi: 10.1186/s43556-022-00112-0. Mol Biomed. 2022. PMID: 36536188 Free PMC article. Review.
-
The Role of Ubiquitination in Regulating Embryonic Stem Cell Maintenance and Cancer Development.Int J Mol Sci. 2019 May 30;20(11):2667. doi: 10.3390/ijms20112667. Int J Mol Sci. 2019. PMID: 31151253 Free PMC article. Review.
-
Traceless Staudinger ligation enabled parallel synthesis of proteolysis targeting chimera linker variants.Chem Commun (Camb). 2021 Jan 25;57(8):1026-1029. doi: 10.1039/d0cc05395c. Epub 2021 Jan 6. Chem Commun (Camb). 2021. PMID: 33406191 Free PMC article.
-
Applications and Limitations of Oxime-Linked "Split PROTACs".Chembiochem. 2022 Sep 16;23(18):e202200275. doi: 10.1002/cbic.202200275. Epub 2022 Jul 28. Chembiochem. 2022. PMID: 35802347 Free PMC article.
-
Chronological Analysis of First-in-Class Drugs Approved from 2011 to 2022: Their Technological Trend and Origin.Pharmaceutics. 2023 Jun 22;15(7):1794. doi: 10.3390/pharmaceutics15071794. Pharmaceutics. 2023. PMID: 37513981 Free PMC article.
References
-
- Bingol B, Tea JS, Phu L, Reichelt M, Bakalarski CE, Song Q, Foreman O, Kirkpatrick DS, Sheng M. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature. 2014;510:370–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

