Suppressed microRNA-96 inhibits iNOS expression and dopaminergic neuron apoptosis through inactivating the MAPK signaling pathway by targeting CACNG5 in mice with Parkinson's disease
- PMID: 30486773
- PMCID: PMC6263543
- DOI: 10.1186/s10020-018-0059-9
Suppressed microRNA-96 inhibits iNOS expression and dopaminergic neuron apoptosis through inactivating the MAPK signaling pathway by targeting CACNG5 in mice with Parkinson's disease
Abstract
Background: There have been a number of reports implicating the association of microRNAs (miRs) and the MAPK signaling pathway with the dopaminergic neuron, which is involved in the development of Parkinson's disease (PD). The present study was conducted with aims of exploring the role of miR-96 in the activation of iNOS and apoptosis of dopaminergic neuron through the MAPK signaling pathway in mice with PD.
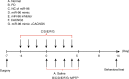
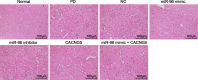
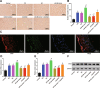
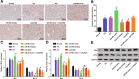
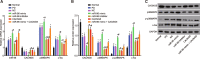
Methods: The miR and the differentially expressed gene in PD were screened out and the relationship between them was verified. A mouse model of PD induced by MPTP and was then constructed and treated with miR-96 mimic/inhibitor and CACNG5 overexpression plasmid to extract nigral dopaminergic neuron for the purpose of detecting the effect of miR-96 on PD. The TH and iNOS positive neuronal cells, the apoptotic neuronal cells by TUNEL staining, and expression of miR-96, CACNG5, iNOS, p38MAPK, p-p38MAPK, c-Fos, Bax, and Bcl-2 in substantia nigra dopaminergic neuronal tissues were evaluated.
Results: The results obtained from the aforementioned procedure were then verified by cell culture of the SH-SY5Y cells, followed by treatment with miR-96 mimic/inhibitor, CACNG5 overexpression plasmid and the inhibitor of the MAPK signaling pathway. CACNG5 was confirmed as a target gene of miR-96. The inhibition of miR-96 resulted in a substantial increase in nigral cells, TH positive cells and expression of CACNG5 and Bcl-2 in nigral dopaminergic neuronal tissues, and a decrease in iNOS positive cells, apoptotic neuronal cells, and expression of iNOS, p38MAPK, p-p38MAPK, c-Fos, and Bax.
Conclusion: The above results implicated that the downregulation of miR-96 inhibits the activation of iNOS and apoptosis of dopaminergic neuron through the blockade of the MAPK signaling pathway by promoting CACNG5 in mice with PD.
Keywords: CACNG5; Dopaminergic neuron; Inducible nitric oxide synthase; MAPK signaling pathway; MicroRNA-96; Parkinson’s disease.
Conflict of interest statement
Ethics approval
The present study was performed in strict accordance with the ethics committee of China-Japan Union Hospital, Jilin University. All efforts were made to minimize the number of animals used and their suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

