Detecting neural assemblies in calcium imaging data
- PMID: 30486809
- PMCID: PMC6262979
- DOI: 10.1186/s12915-018-0606-4
Detecting neural assemblies in calcium imaging data
Erratum in
-
Correction to: Detecting neural assemblies in calcium imaging data.BMC Biol. 2019 Mar 6;17(1):21. doi: 10.1186/s12915-019-0644-6. BMC Biol. 2019. PMID: 30841881 Free PMC article.
Abstract
Background: Activity in populations of neurons often takes the form of assemblies, where specific groups of neurons tend to activate at the same time. However, in calcium imaging data, reliably identifying these assemblies is a challenging problem, and the relative performance of different assembly-detection algorithms is unknown.
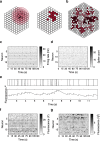
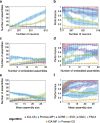
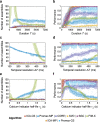
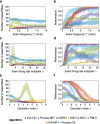
Results: To test the performance of several recently proposed assembly-detection algorithms, we first generated large surrogate datasets of calcium imaging data with predefined assembly structures and characterised the ability of the algorithms to recover known assemblies. The algorithms we tested are based on independent component analysis (ICA), principal component analysis (Promax), similarity analysis (CORE), singular value decomposition (SVD), graph theory (SGC), and frequent item set mining (FIM-X). When applied to the simulated data and tested against parameters such as array size, number of assemblies, assembly size and overlap, and signal strength, the SGC and ICA algorithms and a modified form of the Promax algorithm performed well, while PCA-Promax and FIM-X did less well, for instance, showing a strong dependence on the size of the neural array. Notably, we identified additional analyses that can improve their importance. Next, we applied the same algorithms to a dataset of activity in the zebrafish optic tectum evoked by simple visual stimuli, and found that the SGC algorithm recovered assemblies closest to the averaged responses.
Conclusions: Our findings suggest that the neural assemblies recovered from calcium imaging data can vary considerably with the choice of algorithm, but that some algorithms reliably perform better than others. This suggests that previous results using these algorithms may need to be reevaluated in this light.
Keywords: Clustering; Population coding; Spontaneous activity.
Conflict of interest statement
Ethics approval and consent to participate
All experiments were performed with approval from The University of Queensland Animal Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

