A process and mechanism of action evaluation of the effect of early and intensive nutrition care, delivered via telephone or mobile application, on quality of life in people with upper gastrointestinal cancer: a study protocol
- PMID: 30486814
- PMCID: PMC6262954
- DOI: 10.1186/s12885-018-5089-8
A process and mechanism of action evaluation of the effect of early and intensive nutrition care, delivered via telephone or mobile application, on quality of life in people with upper gastrointestinal cancer: a study protocol
Abstract
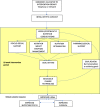
Background: Cancers of the upper gastrointestinal tract commonly result in malnutrition, which increases morbidity and mortality. Current nutrition best practice lacks a mechanism to provide early and intensive nutrition support to these patients. A 3-arm parallel randomised controlled trial is testing the provision of a tailored, nutritional counselling intervention delivered using a synchronous, telephone-based approach or an asynchronous, mobile application-based approach to address this problem. This protocol outlines the design and methods that will be used to undertake an evaluation of the implementation process, which is imperative for successful replication and dissemination.
Methods: A concurrent triangulation mixed methods comparative analysis will be undertaken. The nutrition intervention will be provided using best practice behaviour change techniques and communicated either via telephone or via mHealth. The implementation outcomes that will be measured are: fidelity to the nutrition intervention protocol and to the delivery approach; engagement; acceptability and contextual factors. Qualitative data from recorded telephone consultations and written messages will be analysed through a coding matrix against the behaviour change techniques outlined in the standard operating procedure, and also thematically to determine barriers and enablers. Negative binomial regression will be used to test for predictive relationships between intervention components with health-related quality of life and nutrition outcomes. Post-intervention interviews with participants and health professionals will be thematically analysed to determine the acceptability of delivery approaches. NVivo 11 Pro software will be used to code for thematic analysis. STATA version 15 will be used to perform quantitative analysis.
Discussion: The findings of this process evaluation will provide evidence of the core active ingredients that enable the implementation of best practice nutrition intervention for people with upper gastrointestinal cancer. Elucidation of the causal pathways of successful implementation and the important relationship to contextual delivery are anticipated. With this information, a strategy for sustained implementation across broader settings will be developed which impact the quality of life and nutritional status of individuals with upper gastrointestinal cancer.
Trial registration: 27th January 2017 Australian and New Zealand Clinical Trial Registry ( ACTRN12617000152325 ).
Keywords: Behaviour change; Dietetic intervention; Effectiveness; Engagement; Oesophagogastric; Pancreatic cancer; Process evaluation; mHealth.
Conflict of interest statement
Ethics approval and consent to participate
This study has undergone full ethical review by the Monash Health Human Research Ethics Committee and was approved 14th October 2016 (HREC/16/MonH/290). Site-specific authorisation has been granted for all sites (Monash Health, Cabrini Health and Peninsula Health). Participants will provide informed verbal consent to participate in the study, which is the approved consent process by the main ethics committee (Monash Health Human Research Ethics) and the other sites (Cabrini health and Peninsula Health). The decision to use verbal consent rather than written consent was based on feedback from consumer representatives who sit on the study advisory committee. Written informed consent will be sought to access participant data from the Medicare and Pharmaceutical Benefits Scheme databases. Health professionals will provide written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- D BJ, Susan A, L DW, M HJ, Teresa B, A IE, Marina R. Evidence based practice guidelines for the nutritional management of cancer cachexia. Nutr Diet. 2006;63(s2):S3–S32. doi: 10.1111/j.1747-0080.2006.00099.x. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

