Airway epithelial cells exposed to wildfire smoke extract exhibit dysregulated autophagy and barrier dysfunction consistent with COPD
- PMID: 30486816
- PMCID: PMC6263553
- DOI: 10.1186/s12931-018-0945-2
Airway epithelial cells exposed to wildfire smoke extract exhibit dysregulated autophagy and barrier dysfunction consistent with COPD
Abstract
Background: Individuals with respiratory disease are being increasingly exposed to wildfire smoke as populations encroach further into forested regions and climate change continues to bring higher temperatures with lower rainfall. Frequent exposures have significant potential to accelerate conditions such as chronic obstructive pulmonary disease (COPD) which is characterised by an exaggerated inflammatory response to environmental stimuli. Here we employ models of human airway epithelium exposed to wildfire smoke-extract (WFSE) to examine modulation in airway epithelial cell (AEC) survival, fragility and barrier function.
Methods: Submerged cultures of small airway epithelial cells (SAEC) and differentiated air-liquid interface (ALI) cultures of primary bronchial AEC (bAEC) were treated for 1-24 h with 1-10% WFSE generated from plant species found in the Australian bushland. Autophagy (LC3-II and Sequestosome), apoptosis (Poly-(ADP)-Ribose Polymerase (PARP) cleavage) and tight junction proteins were measured using western blot. Barrier function was assessed via permeability of fluorescein tracers and measuring trans-epithelial electrical resistance. The production of IL-6 was assessed using ELISA.
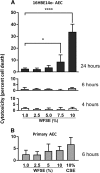
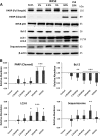
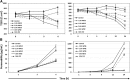
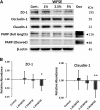
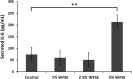
Results: Primary epithelial models exposed to WFSE exhibited a significant blockade in autophagy as evidenced by an increase in LC3-II coupled with a concomitant elevation in Sequestosome abundance. These exposures also induced significant PARP cleavage indicative of apoptotic changes. ALI cultures of bAEC treated with 5% WFSE demonstrated barrier dysfunction with significant increases in paracellular molecular permeability and ionic conductance, and a reduction in the abundance of the tight junction proteins ZO-1 and Claudin-1. These cultures also exhibited increased IL-6 secretion consistent with the aberrant and pro-inflammatory repair response observed in the COPD airways. Further, blocks in autophagy and barrier disruption were significantly elevated in response to WFSE in comparison to similar exposures with cigarette smoke-extract.
Conclusion: WFSE inhibits autophagic flux and induces barrier dysfunction in the airway epithelium. As autophagy is a central regulator of cellular repair, viability, and inflammation, targeting the block in autophagic flux may ameliorate the consequences of wildfire smoke-exposure for individuals with pre-existing respiratory conditions.
Keywords: Airway epithelium; Barrier function; COPD; Cigarette smoke; Environmental health; Exacerbation; Wildfire.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Royal Adelaide Human Ethics Committee (Protocol #R20020811). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Zinc deficiency as a codeterminant for airway epithelial barrier dysfunction in an ex vivo model of COPD.Int J Chron Obstruct Pulmon Dis. 2017 Dec 5;12:3503-3510. doi: 10.2147/COPD.S149589. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 29255357 Free PMC article.
-
Andrographolide simultaneously augments Nrf2 antioxidant defense and facilitates autophagic flux blockade in cigarette smoke-exposed human bronchial epithelial cells.Toxicol Appl Pharmacol. 2018 Dec 1;360:120-130. doi: 10.1016/j.taap.2018.10.005. Epub 2018 Oct 4. Toxicol Appl Pharmacol. 2018. PMID: 30291937
-
The uncoupling of autophagy and zinc homeostasis in airway epithelial cells as a fundamental contributor to COPD.Am J Physiol Lung Cell Mol Physiol. 2017 Sep 1;313(3):L453-L465. doi: 10.1152/ajplung.00083.2017. Epub 2017 Jun 8. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28596293
-
Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure.Am J Respir Cell Mol Biol. 2018 Feb;58(2):157-169. doi: 10.1165/rcmb.2017-0200TR. Am J Respir Cell Mol Biol. 2018. PMID: 28933915 Review.
-
"Air That Once Was Breath" Part 1: Wildfire-Smoke-Induced Mechanisms of Airway Inflammation - "Climate Change, Allergy and Immunology" Special IAAI Article Collection: Collegium Internationale Allergologicum Update 2023.Int Arch Allergy Immunol. 2024;185(6):600-616. doi: 10.1159/000536578. Epub 2024 Mar 7. Int Arch Allergy Immunol. 2024. PMID: 38452750 Free PMC article. Review.
Cited by
-
Modelling bronchial epithelial-fibroblast cross-talk in idiopathic pulmonary fibrosis (IPF) using a human-derived in vitro air liquid interface (ALI) culture.Sci Rep. 2024 Jan 2;14(1):240. doi: 10.1038/s41598-023-50618-y. Sci Rep. 2024. PMID: 38168149 Free PMC article.
-
Physicochemical and toxicological properties of wood smoke particulate matter as a function of wood species and combustion condition.J Hazard Mater. 2023 Jan 5;441:129874. doi: 10.1016/j.jhazmat.2022.129874. Epub 2022 Sep 1. J Hazard Mater. 2023. PMID: 36084462 Free PMC article.
-
The Multifaceted Roles of Autophagy in Infectious, Obstructive, and Malignant Airway Diseases.Biomedicines. 2022 Aug 11;10(8):1944. doi: 10.3390/biomedicines10081944. Biomedicines. 2022. PMID: 36009490 Free PMC article. Review.
-
p62/SQSTM1 accumulation due to degradation inhibition and transcriptional activation plays a critical role in silica nanoparticle-induced airway inflammation via NF-κB activation.J Nanobiotechnology. 2020 May 19;18(1):77. doi: 10.1186/s12951-020-00634-1. J Nanobiotechnology. 2020. PMID: 32429946 Free PMC article.
-
Prothrombotic Biomarkers Are Not Altered by Wood Smoke: A Pilot Controlled Exposure Study.FASEB Bioadv. 2025 Jul 8;7(7):e70038. doi: 10.1096/fba.2025-00125. eCollection 2025 Jul. FASEB Bioadv. 2025. PMID: 40641849 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

