Duplex fluorescence melting curve analysis as a new tool for rapid detection and differentiation of genotype I, II and Bartha-K61 vaccine strains of pseudorabies virus
- PMID: 30486818
- PMCID: PMC6264625
- DOI: 10.1186/s12917-018-1697-4
Duplex fluorescence melting curve analysis as a new tool for rapid detection and differentiation of genotype I, II and Bartha-K61 vaccine strains of pseudorabies virus
Abstract
Background: Recently, pseudorabies (PR) outbreaks have been reported in a large number of swine herds vaccinated with the Bartha-K61 vaccine in China, the current pseudorabies virus (PRV) belonging to Genotype II is differential genetically from Bartha-K61 vaccine belonging to Genotype I. Furthermore, it has been proved that the Bartha-K61 vaccine cannot provide sufficient protection against the current PRVs in China. Therefore, the accurate and rapid identification of PRVs is essential. The objective of this study is to develop a duplex fluorescence melting curve analysis (FMCA) capable of rapid, simple, high-throughput differentiation of Chinese, European/American and Bartha-K61 vaccine strains of PRV.
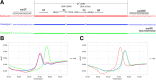
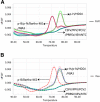
Results: Primers 6F/6R and probes P1/P2, combined with three recombinant plasmids p-B (Bartha-K61), p-N (Genotype I), and p-H (Genotype II), were used to establish the Bicolor FMCA. FAM Tm values (probe P1) and HEX (probe P2) channels of p-B were used as reference values. Tm differences (ΔTm) between detected samples and reference plasmid p-B were calculated in each channel. Bartha-K61 vaccine samples had ΔTm values of ±1 °C in both FAM and HEX channels, Genotype I samples had ΔTm values of ±1 °C in the FAM channel and 4.38 ± 1 °C in the HEX channel, and Genotype II samples had ΔTm values of 6.52 ± 1 °C in the FAM channel and 4.38 ± 1 °C in the HEX channel. The minimum detection limit of the duplex FMCA was approximately 1 × 100 copies per reaction for p-B, p-N, and p-H. The duplex FMCA technique was used to detect and different 198 suspected clinical samples, of which 18 (9%) were positive for Genotype II strains and eight (4%) were positive for Bartha-K61 vaccine strains, and the results were compared with sequencing and phylogenetic analyses, which confirmed that the Bicolor FMCA worked correctly for all samples.
Conclusions: In this study, we developed a duplex FMCA of dual-labeled, self-quenched probes that was performed for rapid detection and differentiation of Genotype I, II and Bartha-K61 vaccine strains of PRV. The duplex FMCA was rapid, simple, and high-throughput, and will likely prove useful for molecular epidemiological investigations and pathogen surveillance of PRV.
Keywords: Duplex FMCA; Genotyping; PRV.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
A gD&gC-substituted pseudorabies virus vaccine strain provides complete clinical protection and is helpful to prevent virus shedding against challenge by a Chinese pseudorabies variant.BMC Vet Res. 2019 Jan 3;15(1):2. doi: 10.1186/s12917-018-1766-8. BMC Vet Res. 2019. PMID: 30606159 Free PMC article.
-
A triplex real-time PCR for differential detection of classical, variant and Bartha-K61 vaccine strains of pseudorabies virus.Arch Virol. 2016 Sep;161(9):2425-30. doi: 10.1007/s00705-016-2925-5. Epub 2016 Jun 17. Arch Virol. 2016. PMID: 27316441
-
A novel gE-deleted pseudorabies virus (PRV) provides rapid and complete protection from lethal challenge with the PRV variant emerging in Bartha-K61-vaccinated swine population in China.Vaccine. 2014 Jun 5;32(27):3379-85. doi: 10.1016/j.vaccine.2014.04.035. Epub 2014 Apr 30. Vaccine. 2014. PMID: 24793946
-
The Attenuated Pseudorabies Virus Vaccine Strain Bartha K61: A Brief Review on the Knowledge Gathered During 60 Years of Research.Pathogens. 2020 Oct 27;9(11):897. doi: 10.3390/pathogens9110897. Pathogens. 2020. PMID: 33121171 Free PMC article. Review.
-
A Review of Pseudorabies Virus Variants: Genomics, Vaccination, Transmission, and Zoonotic Potential.Viruses. 2022 May 9;14(5):1003. doi: 10.3390/v14051003. Viruses. 2022. PMID: 35632745 Free PMC article. Review.
Cited by
-
Advances in molecular epidemiology and detection methods of pseudorabies virus.Discov Nano. 2025 Feb 24;20(1):45. doi: 10.1186/s11671-025-04217-7. Discov Nano. 2025. PMID: 39992589 Free PMC article. Review.
-
Pseudorabies Virus: From Pathogenesis to Prevention Strategies.Viruses. 2022 Jul 27;14(8):1638. doi: 10.3390/v14081638. Viruses. 2022. PMID: 36016260 Free PMC article. Review.
-
Rapid Detection of SLCO1B1 Polymorphisms Using Duplex Fluorescence Melting Curve Analysis: Implications for Personalized Drug Dosing in Clinical Settings.Drug Des Devel Ther. 2024 Nov 3;18:4889-4899. doi: 10.2147/DDDT.S491972. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39512267 Free PMC article.
-
Application of Duplex Fluorescence Melting Curve Analysis (FMCA) to Identify Canine Parvovirus Type 2 Variants.Front Microbiol. 2019 Mar 5;10:419. doi: 10.3389/fmicb.2019.00419. eCollection 2019. Front Microbiol. 2019. PMID: 30891024 Free PMC article.
References
-
- Mettenleiter TC. Pseudorabies virus A2 - Mahy, Brian W.J. In: Regenmortel MHVV, editor. Encyclopedia of Virology (Third Edition). Edn. Oxford: Academic Press; 2008. pp. 341–351.
-
- Beran GW, Davies EB, Arambulo PV, 3rd, Will LA, Hill HT, Rock DL. Persistence of pseudorabies virus in infected swine. J Am Vet Med Assoc. 1980;176(10 Pt 1):998–1000. - PubMed
MeSH terms
Substances
Grants and funding
- 2016A040403084/Science and Technology Planning Project of Guangdong Province, China
- 2015A030401066/Science and Technology Planning Project of Guangdong Province, China
- 2017A020208030/Science and Technology Planning Project of Guangdong Province, China
- 2016B020234006/Science and Technology Planning Project of Guangdong Province, China
- 2016A040403085/Science and Technology Planning Project of Guangdong Province, China
LinkOut - more resources
Full Text Sources
Research Materials

