Evaluation and optimization of a conventional SPCE for FMD post-vaccination monitoring
- PMID: 30486820
- PMCID: PMC6260702
- DOI: 10.1186/s12917-018-1686-7
Evaluation and optimization of a conventional SPCE for FMD post-vaccination monitoring
Abstract
Background: Foot-and-mouth disease (FMD) can be controlled by either stamping out or vaccination, a choice which depends on both the economic importance of the livestock sector as well as the disease status. In FMD-free countries with vaccination, such as Korea, vaccination programs should guarantee prevention against transmission of FMD. Monitoring of vaccination programs is also essential for ensuring sufficient coverage that will limit the transmission of FMDV. There are several methods to screen FMD virus (FMDV) structural protein (SP) antibodies including SPCE (Solid-phase competitive ELISA), LPBE (Liquid-phase blocking ELISA), and VNT (Virus neutralization test). Among these, SPCE is widely used for serological monitoring since VNT-the gold standard method-has certain practical limitations, such as high costs in terms of time and labor. However, whether SPCE can ensure the vaccination status of individual animals and whole farms is unclear. In this study, SPCE, LPBE and VNT were compared with respect to correlation with each other and sensitivity at commercial pig farms.
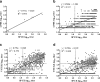
Results: The positive results obtained by PrioCHECK SPCE differed from those obtained by LPBE and VNT. The sensitivity of SPCE relative to those of the other tests was fairly low. The raw data of SPCE were most highly correlated with those of VNT with XJ strain, while their positivity and negativity were most highly correlated with LPBE. The results of ROC analysis proposed new cut-off for PrioCHECK SPCE higher than the previous 50% inhibition.
Conclusions: The high false positive rate of PrioCHECK SPCE suggested that high seropositivity by SPCE may not guarantee a true vaccination coverage. Adjusting the cut-off percentage (%) inhibition value for SPCE is needed to address this problem, and it is highly recommended that routine FMDV serological monitoring programs using PrioCHECK SPCE should be combined with alternative methods such as LPBE or VNT.
Keywords: Foot-and-mouth disease; Liquid-phase blocking ELISA; Serological monitoring; Solid-phase competitive ELISA; Vaccination coverage; Virus neutralization test.
Conflict of interest statement
Ethics approval
The authors confirm that the work complies with the ethical policies of the journal. The work was approved by the Institutional Animal Care and Use Committee of the Konkuk University (Seoul, Republic of Korea) under Authority No.: KU18107. The authors obtained approval for conducting the study in written consents from owners of all farms.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Evaluating serological tests for foot-and-mouth disease while accounting for different serotypes and uncertain vaccination status.Prev Vet Med. 2023 May;214:105889. doi: 10.1016/j.prevetmed.2023.105889. Epub 2023 Mar 7. Prev Vet Med. 2023. PMID: 36906937
-
Comparative evaluation of serological assays for detecting antibodies against structural proteins elicited by foot-and-mouth disease virus vaccines of serotypes O, A, Asia 1 and SAT 2.Vet Immunol Immunopathol. 2025 Aug;286:110978. doi: 10.1016/j.vetimm.2025.110978. Epub 2025 Jul 23. Vet Immunol Immunopathol. 2025. PMID: 40714574
-
Evaluation of commercial quadrivalent foot-and-mouth disease vaccines against east African virus strains reveals limited immunogenicity and duration of protection.Vaccine. 2024 Dec 2;42(26):126325. doi: 10.1016/j.vaccine.2024.126325. Epub 2024 Sep 12. Vaccine. 2024. PMID: 39270355
-
Estimating the protection afforded by foot-and-mouth disease vaccines in the laboratory.Vaccine. 2019 Sep 3;37(37):5515-5524. doi: 10.1016/j.vaccine.2019.07.102. Epub 2019 Aug 9. Vaccine. 2019. PMID: 31405637 Review.
-
The position of the Dutch Farmers' Union on lessons learned and future prevention and control of foot and mouth disease.Rev Sci Tech. 2002 Dec;21(3):839-50. doi: 10.20506/rst.21.3.1372. Rev Sci Tech. 2002. PMID: 12523719 Review.
Cited by
-
Development of Monoclonal Antibody Specific to Foot-and-Mouth Disease Virus Type A for Serodiagnosis.Pathogens. 2019 Dec 17;8(4):301. doi: 10.3390/pathogens8040301. Pathogens. 2019. PMID: 31861046 Free PMC article.
-
Advances in the Diagnosis of Foot-and-Mouth Disease.Front Vet Sci. 2020 Aug 21;7:477. doi: 10.3389/fvets.2020.00477. eCollection 2020. Front Vet Sci. 2020. PMID: 32974392 Free PMC article. Review.
-
Current trends and challenges in the management of foot and mouth disease in Saudi Arabia: A review.Open Vet J. 2025 May;15(5):1907-1933. doi: 10.5455/OVJ.2025.v15.i5.6. Epub 2025 May 31. Open Vet J. 2025. PMID: 40557100 Free PMC article. Review.
References
-
- Bergmann IE, de Mello PA, Neitzert E, Beck E, Gomes I. Diagnosis of persistent aphthovirus infection and its differentiation from vaccination response in cattle by use of enzyme-linked immunoelectrotransfer blot analysis with bioengineered nonstructural viral antigens. Am J Vet Res. 1993;54(6):825–831. - PubMed
-
- Neitzert E, Beck E, de Mello PA, Gomes I, Bergmann IE. Expression of the aphthovirus RNA polymerase gene in Escherichia coli and its use together with other bioengineered nonstructural antigens in detection of late persistent infections. Virology. 1991;184(2):799–804. doi: 10.1016/0042-6822(91)90456-L. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

