Patient and observer reported outcome measures to evaluate health-related quality of life in inherited metabolic diseases: a scoping review
- PMID: 30486833
- PMCID: PMC6263554
- DOI: 10.1186/s13023-018-0953-9
Patient and observer reported outcome measures to evaluate health-related quality of life in inherited metabolic diseases: a scoping review
Abstract
Background: Health-related Quality of Life (HrQoL) is a multidimensional measure, which has gained clinical and social relevance. Implementation of a patient-centred approach to both clinical research and care settings, has increased the recognition of patient and/or observer reported outcome measures (PROMs or ObsROMs) as informative and reliable tools for HrQoL assessment. Inherited Metabolic Diseases (IMDs) are a group of heterogeneous conditions with phenotypes ranging from mild to severe and mostly lacking effective therapies. Consequently, HrQoL evaluation is particularly relevant.
Objectives: We aimed to: (1) identify patient and/or caregiver-reported HrQoL instruments used among IMDs; (2) identify the main results of the application of each HrQoL tool and (3) evaluate the main limitations of HrQoL instruments and study design/methodology in IMDs.
Methods: A scoping review was conducted using methods outlined by Arksey and O'Malley. Additionally, we critically analysed each article to identify the HrQoL study drawbacks.
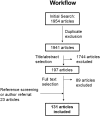
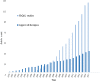
Results: Of the 1954 studies identified, 131 addressed HrQoL of IMDs patients using PROMs and/or ObsROMs, both in observational or interventional studies. In total, we identified 32 HrQoL instruments destined to self- or proxy-completion; only 2% were disease-specific. Multiple tools (both generic and disease-specific) proved to be responsive to changes in HrQoL; the SF-36 and PedsQL questionnaires were the most frequently used in the adult and pediatric populations, respectively. Furthermore, proxy data often demonstrated to be a reliable approach complementing self-reported HrQoL scores. Nevertheless, numerous limitations were identified especially due to the rarity of these conditions.
Conclusions: HrQoL is still not frequently assessed in IMDs. However, our results show successful examples of the use of patient-reported HrQoL instruments in this field. The importance of HrQoL measurement for clinical research and therapy development, incites to further research in HrQoL PROMs' and ObsROMs' creation and validation in IMDs.
Keywords: Health-related quality of life (HrQoL); Inherited metabolic disease(s) (IMD(s)); Observer reported outcome measures (ObsROMs); Patient reported outcome measures (PROMs); Quality of life (QoL).
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
V.R.F is the president and founder of the Portuguese Association for Congenital Disorders of Glycosylation. A.R. is the president of Glycomine, Inc. The costs regarding this publication were covered by Glycomine, Inc. All other authors declare no conflict of interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
A comprehensive systematic review of health-related quality of life measures in short stature paediatric patients.Endocrine. 2024 Nov;86(2):478-504. doi: 10.1007/s12020-024-03938-6. Epub 2024 Jul 17. Endocrine. 2024. PMID: 39017834 Free PMC article.
-
Risk factors for impaired health-related quality of life in a cohort of pediatric patients with inborn metabolic diseases.Eur J Pediatr. 2022 Mar;181(3):1063-1070. doi: 10.1007/s00431-021-04300-y. Epub 2021 Oct 31. Eur J Pediatr. 2022. PMID: 34718865 Free PMC article.
-
Patient-Reported Outcome Measures for Health-Related Quality of Life in Patients With Acne Vulgaris: A Systematic Review of Measure Development and Measurement Properties.JAMA Dermatol. 2022 Aug 1;158(8):900-911. doi: 10.1001/jamadermatol.2022.2260. JAMA Dermatol. 2022. PMID: 35731537 Free PMC article.
-
Measuring health related quality of life (HRQoL) in Lysosomal Storage Disorders (LSDs): a rapid scoping review of available tools and domains.Orphanet J Rare Dis. 2024 Jul 4;19(1):252. doi: 10.1186/s13023-024-03256-0. Orphanet J Rare Dis. 2024. PMID: 38965628 Free PMC article.
-
Validation study of the prototype of a disease-specific index measure for health-related quality of life in dementia.Health Qual Life Outcomes. 2012 Sep 25;10:118. doi: 10.1186/1477-7525-10-118. Health Qual Life Outcomes. 2012. PMID: 23009579 Free PMC article. Clinical Trial.
Cited by
-
The impact of a child's inborn error of metabolism: the parents' perspectives on restrictions, discrimination, family planning, and emergency management.Orphanet J Rare Dis. 2024 Aug 26;19(1):313. doi: 10.1186/s13023-024-03315-6. Orphanet J Rare Dis. 2024. PMID: 39187849 Free PMC article.
-
The Uneven Effect of Rare Diseases on Functional Status and Work Capacity.Healthcare (Basel). 2025 Mar 8;13(6):594. doi: 10.3390/healthcare13060594. Healthcare (Basel). 2025. PMID: 40150443 Free PMC article.
-
Key patient-reported outcomes in children and adolescents with intoxication-type inborn errors of metabolism: an international Delphi-based consensus.Orphanet J Rare Dis. 2022 Jan 29;17(1):26. doi: 10.1186/s13023-022-02183-2. Orphanet J Rare Dis. 2022. PMID: 35093149 Free PMC article.
-
Exploring Partners, Parenting and Pregnancy Thinking in Late Adolescents and Young Adults with Inherited Metabolic Disorders.Pediatr Rep. 2025 May 13;17(3):56. doi: 10.3390/pediatric17030056. Pediatr Rep. 2025. PMID: 40407581 Free PMC article.
-
Qualitative interviews to improve patient-reported outcome measures in late-onset Pompe disease: the patient perspective.Orphanet J Rare Dis. 2021 Oct 12;16(1):428. doi: 10.1186/s13023-021-02067-x. Orphanet J Rare Dis. 2021. PMID: 34641935 Free PMC article.
References
-
- Bonner N, Abetz-Webb L, Renault L, Caballero T, Longhurst H, Maurer M, et al. Development and content validity testing of a patient-reported outcomes questionnaire for the assessment of hereditary angioedema in observational studies. Health Qual Life Outcomes. 2015;13:1–15. doi: 10.1186/s12955-015-0292-7. - DOI - PMC - PubMed
-
- Healthy People 2020 . Health-Related Quality of Life and Well-Being. 2010.
-
- FDA. Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims Clinical/Medical Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Health Qual Life Outcomes. 2006;4:79. 10.1186/1477-7525-4-79. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

