Statistical analysis plan for the control of blood pressure and risk attenuation-rural Bangladesh, Pakistan, Sri Lanka (COBRA-BPS) trial: a cluster randomized trial for a multicomponent intervention versus usual care in hypertensive patients
- PMID: 30486858
- PMCID: PMC6263546
- DOI: 10.1186/s13063-018-3022-8
Statistical analysis plan for the control of blood pressure and risk attenuation-rural Bangladesh, Pakistan, Sri Lanka (COBRA-BPS) trial: a cluster randomized trial for a multicomponent intervention versus usual care in hypertensive patients
Abstract
Background: In rural south Asia, hypertension remains a significant public health issue with sub-optimal blood pressure (BP) control rates. The goal of the trial is to evaluate the effectiveness and cost-effectiveness of a multicomponent intervention (MCI) compared to usual care on lowering BP among adults with hypertension in rural south-Asian communities. This article describes the statistical analysis plan for the primary and secondary objectives related to intervention effectiveness based on clinical and patient-reported endpoints.
Methods/design: The study is a cluster randomized trial which will enroll 2550 participants aged ≥ 40 years with hypertension from rural communities in Bangladesh, Pakistan, and Sri Lanka. The unit of randomization is a cluster defined by 250-300 households. Thirty clusters, 10 from each country, are randomized in a 1:1 ratio to either MCI or usual care, stratified by country and their distance from the clinic. All participants will be assessed every six months over a two-year period after baseline with measurements of systolic and diastolic BP, antihypertensive and statin medication use, medication adherence, physical activity level, anthropometric parameters, smoking status, and dietary habits. The primary objective is to assess the effectiveness of MCI as compared with usual care in terms of mean change in systolic BP from baseline to final follow-up at two years. The primary outcome will be modelled using a generalized linear mixed-model for repeated measures based on a participant-level analysis. The model will include cluster random-effects and will use a non-independence residual correlation matrix to account for repeated measures on the same participant. Sensitivity analyses for the primary endpoint will be based on multiple imputation as well as pattern mixture model tipping point analyses. Secondary outcomes will be analyzed using the same modeling approach as for the primary outcome, with appropriate distributions within the exponential family and corresponding link functions.
Discussion: The a priori statistical analysis plan will avoid reporting bias and data-driven analysis for the primary and key secondary outcomes. The results of the study will provide evidence of the benefits and risks of the MCI for BP control in rural communities in south Asian countries with low-resourced public health infrastructure.
Trial registration: Clinicaltrials.gov, NCT02657746 . Registered on 14 January 2016.
Keywords: Blood pressure; Cluster randomized trial; Hypertension; Statistical analysis plan.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval for the study has been obtained from the Ethics Review Committee at the Duke-NUS Graduate Medical School, Singapore, International Centre for Diarrhoeal Disease Research, Bangladesh, the Aga Khan University, Pakistan, the University of Kelaniya, Sri Lanka, and the London School of Hygiene and Tropical Medicine, UK. Written informed consent will be obtained from the participants before participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
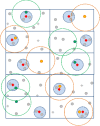
 Multicomponent intervention cluster;
Multicomponent intervention cluster;  Usual care cluster;
Usual care cluster;  Government clinic nearest to the randomized cluster;
Government clinic nearest to the randomized cluster;  Non-randomized cluster;
Non-randomized cluster;  Government clinic outside 10 km radius of randomized cluster. Rectangles represent administrative units. Dotted line surrounding a cluster represents a 10-km radius to the cluster. Dotted line surrounding a government clinic represents a 2-km radium to the clinic. No usual care clusters are within a 10-km radius of MCI clusters. Any usual care cluster should not be nearer to a MCI government clinic than its own; similarly, any MCI cluster should not be nearer to a usual care government clinic than its own
Government clinic outside 10 km radius of randomized cluster. Rectangles represent administrative units. Dotted line surrounding a cluster represents a 10-km radius to the cluster. Dotted line surrounding a government clinic represents a 2-km radium to the clinic. No usual care clusters are within a 10-km radius of MCI clusters. Any usual care cluster should not be nearer to a MCI government clinic than its own; similarly, any MCI cluster should not be nearer to a usual care government clinic than its own
Similar articles
-
Multicomponent intervention versus usual care for management of hypertension in rural Bangladesh, Pakistan and Sri Lanka: study protocol for a cluster randomized controlled trial.Trials. 2017 Jun 12;18(1):272. doi: 10.1186/s13063-017-2018-0. Trials. 2017. PMID: 28606184 Free PMC article. Clinical Trial.
-
Statistical analysis plan for management of hypertension and multiple risk factors to enhance cardiovascular health in Singapore: the SingHypertension pragmatic cluster randomized controlled trial.Trials. 2021 Jan 19;22(1):66. doi: 10.1186/s13063-020-05016-4. Trials. 2021. PMID: 33468225 Free PMC article. Clinical Trial.
-
Budget impact and cost-effectiveness analyses of the COBRA-BPS multicomponent hypertension management programme in rural communities in Bangladesh, Pakistan, and Sri Lanka.Lancet Glob Health. 2021 May;9(5):e660-e667. doi: 10.1016/S2214-109X(21)00033-4. Epub 2021 Mar 19. Lancet Glob Health. 2021. PMID: 33751956 Free PMC article. Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Mobile phone-based interventions for improving adherence to medication prescribed for the primary prevention of cardiovascular disease in adults.Cochrane Database Syst Rev. 2021 Mar 26;3(3):CD012675. doi: 10.1002/14651858.CD012675.pub3. Cochrane Database Syst Rev. 2021. PMID: 33769555 Free PMC article.
Cited by
-
Lowering blood pressure by changing lifestyle through a motivational education program: a cluster randomized controlled trial study protocol.Trials. 2021 Jul 8;22(1):438. doi: 10.1186/s13063-021-05379-2. Trials. 2021. PMID: 34238363 Free PMC article.
-
Effect of a Multicomponent Intervention on Antihypertensive Medication Intensification in Rural South Asia: Post Hoc Analysis of a Cluster RCT.Am J Hypertens. 2021 Sep 22;34(9):981-988. doi: 10.1093/ajh/hpab072. Am J Hypertens. 2021. PMID: 34013966 Free PMC article. Clinical Trial.
-
Study of the psychometric properties of the HLS-EU-12 questionnaire in rural Bangladesh.PLoS One. 2025 May 12;20(5):e0323608. doi: 10.1371/journal.pone.0323608. eCollection 2025. PLoS One. 2025. PMID: 40354386 Free PMC article. Clinical Trial.
-
Associations of physical activity levels, and attitudes towards physical activity with blood pressure among adults with high blood pressure in Bangladesh.PLoS One. 2023 Feb 3;18(2):e0280879. doi: 10.1371/journal.pone.0280879. eCollection 2023. PLoS One. 2023. PMID: 36735692 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

