The presence of anti-nuclear antibodies alone is associated with changes in B cell activation and T follicular helper cells similar to those in systemic autoimmune rheumatic disease
- PMID: 30486869
- PMCID: PMC6263058
- DOI: 10.1186/s13075-018-1752-3
The presence of anti-nuclear antibodies alone is associated with changes in B cell activation and T follicular helper cells similar to those in systemic autoimmune rheumatic disease
Abstract
Background: Diagnosis of systemic autoimmune rheumatic diseases (SARD) relies on the presence of hallmark anti-nuclear antibodies (ANA), many of which can be detected years before clinical manifestations. However, ANAs are also seen in healthy individuals, most of whom will not develop SARD. Here, we examined a unique cohort of asymptomatic ANA+ individuals to determine whether they share any of the cellular immunologic features seen in SARD.
Methods: Healthy ANA- controls and ANA+ (ANA ≥1:160 by immunofluorescence) participants with no SARD criteria, with at least one criterion (undifferentiated connective tissue disease (UCTD)), or meeting SARD classification criteria were recruited. Peripheral blood cellular immunological changes were assessed by flow cytometry and transcript levels of BAFF, interferon (IFN)-induced and plasma cell-expressed genes were quantified by NanoString.
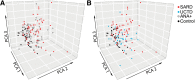
Results: A number of the immunologic abnormalities seen in SARD, including changes in peripheral B (switched memory) and T (iNKT, T regulatory, activated memory T follicular helper) subsets and B cell activation, were also seen in asymptomatic ANA+ subjects and those with UCTD. The extent of these immunologic changes correlated with ANA titer or the number of different specific ANAs produced. Principal component analysis of the cellular data indicated that a significant proportion of asymptomatic ANA+ subjects and subjects with UCTD clustered with patients with early SARD, rather than ANA- healthy controls.
Conclusions: ANA production is associated with altered T and B cell activation even in asymptomatic individuals. Some of the currently accepted cellular features of SARD may be associated with ANA production rather than the immunologic events that cause symptoms in SARD.
Keywords: Anti-nuclear antibodies; B cell; Systemic autoimmune rheumatic disease; T cell.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Research Ethics Boards of the University Health Network (12-5455-BE) and Mount Sinai Hospital, and all participants signed informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Alarcon-Segovia D, Alarcon-Riquelme ME, Cardiel MH, Caeiro F, Massardo L, Villa AR, Pons-Estel BA. Familial aggregation of systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune diseases in 1,177 lupus patients from the GLADEL cohort. Arthritis Rheum. 2005;52(4):1138–1147. doi: 10.1002/art.20999. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

