Induction of cell cycle arrest and inflammatory genes by combined treatment with epigenetic, differentiating, and chemotherapeutic agents in triple-negative breast cancer
- PMID: 30486871
- PMCID: PMC6263070
- DOI: 10.1186/s13058-018-1068-x
Induction of cell cycle arrest and inflammatory genes by combined treatment with epigenetic, differentiating, and chemotherapeutic agents in triple-negative breast cancer
Abstract
Background: A combination of entinostat, all-trans retinoic acid, and doxorubicin (EAD) induces cell death and differentiation and causes significant regression of xenografts of triple-negative breast cancer (TNBC).
Methods: We investigated the mechanisms underlying the antitumor effects of each component of the EAD combination therapy by high-throughput gene expression profiling of drug-treated cells.
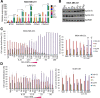
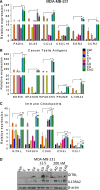
Results: Microarray analysis showed that entinostat and doxorubicin (ED) altered expression of genes related to growth arrest, inflammation, and differentiation. ED downregulated MYC, E2F, and G2M cell cycle genes. Accordingly, entinostat sensitized the cells to doxorubicin-induced growth arrest at G2. ED induced interferon genes, which correlated with breast tumors containing a higher proportion of tumor-infiltrating lymphocytes. ED also increased the expression of immune checkpoint agonists and cancer testis antigens. Analysis of TNBC xenografts showed that EAD enhanced the inflammation score in nude mice. Among the genes differentially regulated between the EAD and ED groups, an all-trans retinoic acid (ATRA)-regulated gene, DHRS3, was induced in EAD-treated xenografts. DHRS3 was expressed at lower levels in human TNBC metastases compared to normal breast or primary tumors. High expression of ED-induced growth arrest and inflammatory genes was associated with better prognosis in TNBC patients.
Conclusions: Entinostat potentiated doxorubicin-mediated cell death and the combination induced inflammatory signatures. The ED-induced immunomodulation may improve immunotherapy. Addition of ATRA to ED may potentiate inflammation and contribute to TNBC regression.
Keywords: Breast cancer; Entinostat; Epigenetic; Immunotherapy.
Conflict of interest statement
Ethics approval and consent to participate
Freshly resected breast tissue of women undergoing reduction mammoplasty, primary tumors, and pleural effusion from women undergoing treatment and from the Rapid Autopsy Program, were provided by the Johns Hopkins Surgical Pathology Department, under protocols approved by the institutional review board. All animal studies were performed according to the guidelines and approval of the Animal Care Committee of the Johns Hopkins School of Medicine, Baltimore.
Consent for publication
All authors approved the final version of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Seewaldt VL, Johnson BS, Parker MB, Collins SJ, Swisshelm K. Expression of retinoic acid receptor beta mediates retinoic acid-induced growth arrest and apoptosis in breast cancer cells. Cell Growth Differ. 1995;6(9):1077–1088. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

