In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells
- PMID: 30486903
- PMCID: PMC6263063
- DOI: 10.1186/s13287-018-1071-2
In vitro and in vivo potentialities for cartilage repair from human advanced knee osteoarthritis synovial fluid-derived mesenchymal stem cells
Abstract
Background: Mesenchymal stem cells (MSCs) are found in synovial fluid (SF) and can easily be harvested during arthrocentesis or arthroscopy. However, SF-MSC characterization and chondrogenicity in collagen sponges have been poorly documented as well as their hypothetical in vivo chondroprotective properties with intra-articular injections during experimental osteoarthritis (OA).
Methods: SF-MSCs were isolated from human SF aspirates in patients suffering from advanced OA undergoing total knee joint replacements. SF-MSCs at passage 2 (P2) were characterized by flow cytometry for epitope profiling. SF-MSCs at P2 were subsequently cultured in vitro to assess their multilineage potentials. To assess their chondrogenicity, SF-MSCs at P4 were seeded in collagen sponges for 4 weeks under various oxygen tensions and growth factors combinations to estimate their gene profile and matrix production. Also, SF-MSCs were injected into the joints in a nude rat anterior cruciate ligament transection (ACLT) to macroscopically and histologically assess their possible chondroprotective properties,.
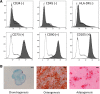
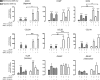
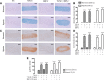
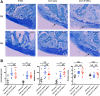
Results: We characterized the stemness (CD73+, CD90+, CD105+, CD34-, CD45-) and demonstrated the multilineage potency of SF-MSCs in vitro. Furthermore, the chondrogenic induction (TGF-ß1 ± BMP-2) of these SF-MSCs in collagen sponges demonstrated a good capacity of chondrogenic gene induction and extracellular matrix synthesis. Surprisingly, hypoxia did not enhance matrix synthesis, although it boosted chondrogenic gene expression (ACAN, SOX9, COL2A1). Besides, intra-articular injections of xenogenic SF-MSCs did exert neither chondroprotection nor inflammation in ACLT-induced OA in the rat knee.
Conclusions: Advanced OA SF-MSCs seem better candidates for cell-based constructs conceived for cartilage defects rather than intra-articular injections for diffuse OA.
Keywords: Cartilage; Collagen sponge; Stem cells; Synovial fluid; Tissue engineering.
Conflict of interest statement
Ethics approval and consent to participate
The clinical protocol was approved by the ethical committee of our university hospital. The experimental study in the rat was approved by our local Ethical Committee for the Animal studies and validated by the French Ministry of National Education, Higher Education and Research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

