Novel insights and therapeutic approaches in idiopathic multicentric Castleman disease
- PMID: 30487129
- PMCID: PMC6265649
- DOI: 10.1182/blood-2018-05-848671
Novel insights and therapeutic approaches in idiopathic multicentric Castleman disease
Abstract
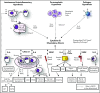
Castleman disease (CD) describes a heterogeneous group of hematologic disorders that share characteristic lymph node histopathology. Patients of all ages present with either a solitary enlarged lymph node (unicentric CD) or multicentric lymphadenopathy (MCD) with systemic inflammation, cytopenias, and life-threatening multiple organ dysfunction resulting from a cytokine storm often driven by interleukin 6 (IL-6). Uncontrolled human herpesvirus-8 (HHV-8) infection causes approximately 50% of MCD cases, whereas the etiology is unknown in the remaining HHV-8-negative/idiopathic MCD cases (iMCD). The limited understanding of etiology, cell types, and signaling pathways involved in iMCD has slowed development of treatments and contributed to historically poor patient outcomes. Here, recent progress for diagnosing iMCD, characterizing etio-pathogenesis, and advancing treatments are reviewed. Several clinicopathological analyses provided the evidence base for the first-ever diagnostic criteria and revealed distinct clinical subtypes: thrombocytopenia, anasarca, fever, reticulin fibrosis/renal dysfunction, organomegaly (iMCD-TAFRO) or iMCD-not otherwise specified (iMCD-NOS), which are both observed all over the world. In 2014, the anti-IL-6 therapy siltuximab became the first iMCD treatment approved by the US Food and Drug Administration, on the basis of a 34% durable response rate; consensus guidelines recommend it as front-line therapy. Recent cytokine and proteomic profiling has revealed normal IL-6 levels in many patients with iMCD and potential alternative driver cytokines. Candidate novel genomic alterations, dysregulated cell types, and signaling pathways have also been identified as candidate therapeutic targets. RNA sequencing for viral transcripts did not reveal novel viruses, HHV-8, or other viruses pathologically associated with iMCD. Despite progress, iMCD remains poorly understood. Further efforts to elucidate etiology, pathogenesis, and treatment approaches, particularly for siltuximab-refractory patients, are needed.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict of interest disclosure: D.C.F. has received research funding from Janssen Pharmaceuticals and serves on the Board of Directors for the Castleman Disease Collaborative Network. Off-label drug use: None disclosed.
Figures


References
-
- Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123(19):2924-2933. - PubMed
-
- Liu AY, Nabel CS, Finkelman BS, et al. Idiopathic multicentric Castleman’s disease: a systematic literature review. Lancet Haematol. 2016;3(4):e163-e175. - PubMed
-
- Munshi N, Mehra M, van de Velde H, Desai A, Potluri R, Vermeulen J. Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk Lymphoma. 2015;56(5):1252-1260. - PubMed
-
- Pria AD, Pinato D, Roe J, Naresh K, Nelson M, Bower M. Relapse of HHV8-positive multicentric Castleman disease following rituximab-based therapy in HIV-positive patients. Blood. 2017;129(15):2143-2147. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

