Diagnosis of Human Immunodeficiency Virus Infection
- PMID: 30487166
- PMCID: PMC6302353
- DOI: 10.1128/CMR.00064-18
Diagnosis of Human Immunodeficiency Virus Infection
Abstract
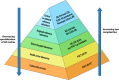
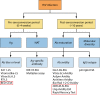
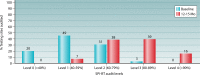
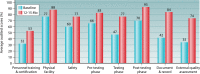
HIV diagnostics have played a central role in the remarkable progress in identifying, staging, initiating, and monitoring infected individuals on life-saving antiretroviral therapy. They are also useful in surveillance and outbreak responses, allowing for assessment of disease burden and identification of vulnerable populations and transmission "hot spots," thus enabling planning, appropriate interventions, and allocation of appropriate funding. HIV diagnostics are critical in achieving epidemic control and require a hybrid of conventional laboratory-based diagnostic tests and new technologies, including point-of-care (POC) testing, to expand coverage, increase access, and positively impact patient management. In this review, we provide (i) a historical perspective on the evolution of HIV diagnostics (serologic and molecular) and their interplay with WHO normative guidelines, (ii) a description of the role of conventional and POC testing within the tiered laboratory diagnostic network, (iii) information on the evaluations and selection of appropriate diagnostics, (iv) a description of the quality management systems needed to ensure reliability of testing, and (v) strategies to increase access while reducing the time to return results to patients. Maintaining the central role of HIV diagnostics in programs requires periodic monitoring and optimization with quality assurance in order to inform adjustments or alignment to achieve epidemic control.
Keywords: CD4; HIV incidence; HIV rapid tests; dried blood spots; drug resistance; early infant diagnosis; enzyme immunoassay; point-of-care testing; quality assurance; viral load.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- UNAIDS. 2017. Fact sheet—World AIDS Day 2017. UNAIDS, Geneva, Switzerland: www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed 2 April 2018.
-
- UNAIDS. 2015. Treatment 2015. UNAIDS, Geneva, Switzerland: www.unaids.org/sites/default/files/media_asset/JC2484_treatment-2015_en_.... Accessed 7 March 2018.
-
- UNAIDS. 2017. Fact sheet July 2017. UNAIDS, Geneva, Switzerland: www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed 11 November 2017.
-
- Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, Wang L, Ou SS, Anderson M, McCauley M, Gamble T, Kumarasamy N, Hakim JG, Kumwenda J, Pilotto JH, Godbole SV, Chariyalertsak S, de Melo MG, Mayer KH, Eshleman SH, Piwowar-Manning E, Makhema J, Mills LA, Panchia R, Sanne I, Gallant J, Hoffman I, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Havlir D, Cohen MS, HPTN 052-ACTG Study Team. 2014. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis 14:281–290. doi: 10.1016/S1473-3099(13)70692-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

