Analysis of a Subacute Sclerosing Panencephalitis Genotype B3 Virus from the 2009-2010 South African Measles Epidemic Shows That Hyperfusogenic F Proteins Contribute to Measles Virus Infection in the Brain
- PMID: 30487282
- PMCID: PMC6364028
- DOI: 10.1128/JVI.01700-18
Analysis of a Subacute Sclerosing Panencephalitis Genotype B3 Virus from the 2009-2010 South African Measles Epidemic Shows That Hyperfusogenic F Proteins Contribute to Measles Virus Infection in the Brain
Abstract
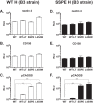
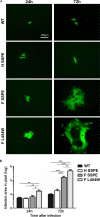
During a measles virus (MeV) epidemic in 2009 in South Africa, measles inclusion body encephalitis (MIBE) was identified in several HIV-infected patients. Years later, children are presenting with subacute sclerosing panencephalitis (SSPE). To investigate the features of established MeV neuronal infections, viral sequences were analyzed from brain tissue samples of a single SSPE case and compared with MIBE sequences previously obtained from patients infected during the same epidemic. Both the SSPE and the MIBE viruses had amino acid substitutions in the ectodomain of the F protein that confer enhanced fusion properties. Functional analysis of the fusion complexes confirmed that both MIBE and SSPE F protein mutations promoted fusion with less dependence on interaction by the viral receptor-binding protein with known MeV receptors. While the SSPE F required the presence of a homotypic attachment protein, MeV H, in order to fuse, MIBE F did not. Both F proteins had decreased thermal stability compared to that of the corresponding wild-type F protein. Finally, recombinant viruses expressing MIBE or SSPE fusion complexes spread in the absence of known MeV receptors, with MIBE F-bearing viruses causing large syncytia in these cells. Our results suggest that alterations to the MeV fusion complex that promote fusion and cell-to-cell spread in the absence of known MeV receptors is a key property for infection of the brain.IMPORTANCE Measles virus can invade the central nervous system (CNS) and cause severe neurological complications, such as MIBE and SSPE. However, mechanisms by which MeV enters the CNS and triggers the disease remain unclear. We analyzed viruses from brain tissue of individuals with MIBE or SSPE, infected during the same epidemic, after the onset of neurological disease. Our findings indicate that the emergence of hyperfusogenic MeV F proteins is associated with infection of the brain. We also demonstrate that hyperfusogenic F proteins permit MeV to enter cells and spread without the need to engage nectin-4 or CD150, known receptors for MeV that are not present on neural cells.
Keywords: central nervous system infections; measles; viral fusion.
Copyright © 2019 American Society for Microbiology.
Figures


References
-
- Ntshoe GM, McAnerney JM, Archer BN, Smit SB, Harris BN, Tempia S, Mashele M, Singh B, Thomas J, Cengimbo A, Blumberg LH, Puren A, Moyes J, van den Heever J, Schoub BD, Cohen C. 2013. Measles outbreak in South Africa: epidemiology of laboratory-confirmed measles cases and assessment of intervention, 2009-2011. PLoS One 8:e55682. doi:10.1371/journal.pone.0055682. - DOI - PMC - PubMed
-
- Watanabe S, Ohno S, Shirogane Y, Suzuki SO, Koga R, Yanagi Y. 2015. Measles virus mutants possessing the fusion protein with enhanced fusion activity spread effectively in neuronal cells, but not in other cells, without causing strong cytopathology. J Virol 89:2710–2717. doi:10.1128/JVI.03346-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

