Effect of Polyelectrolyte Mono- and Bilayer Formation on the Colloidal Stability of Layered Double Hydroxide Nanoparticles
- PMID: 30487401
- PMCID: PMC6316193
- DOI: 10.3390/nano8120986
Effect of Polyelectrolyte Mono- and Bilayer Formation on the Colloidal Stability of Layered Double Hydroxide Nanoparticles
Abstract
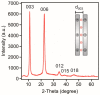
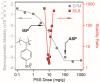
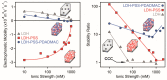
Sequential adsorption of polyelectrolytes on nanoparticles is a popular method to obtain thin films after deposition. However, the effect of polyelectrolyte multilayer formation on the colloidal stability of the nanoparticles has not been studied in detail. In the present work, layered double hydroxides (LDH) were synthesized and interaction with oppositely and like-charged polyelectrolytes was investigated. Electrophoretic and light scattering measurements revealed that colloidal stability of LDH can be tuned by adsorption of poly(styrene sulfonate) (PSS) on the oppositely charged LDH surface in appropriate doses and thus, unstable or stable dispersions can be designed. Negatively charged LDH of adsorbed PSS monolayer was obtained and a poly(diallyldimethyl ammonium chloride) (PDADMAC) second layer was systematically built on the particles. The obtained polyelectrolyte bilayer provided high colloidal stability for the LDH-PSS-PDADMAC dispersions due to the presence of repulsive interparticle forces of electrostatic and steric origin. The results provide crucial quantitative information on designing highly stable particle-polyelectrolyte systems for the preparation of thin films or immobilization of guest substances between the layers for delivery processes.
Keywords: colloidal stability; layered double hydroxide; polyelectrolyte layer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Decher G., Schlenoff J.B. Multilayer Thin Films. Wiley-VCH; Weinheim, Germany: 2002.
-
- Ho T.T.M., Bremmell K.E., Krasowska M., MacWilliams S.V., Richard C.J.E., Stringer D.N., Beattie D.A. In Situ Atr Ftir Spectroscopic Study of the Formation and Hydration of a Fucoidan/Chitosan Polyelectrolyte Multilayer. Langmuir. 2015;31:11249–11259. doi: 10.1021/acs.langmuir.5b01812. - DOI - PubMed
-
- Kotov N.A., Haraszti T., Turi L., Zavala G., Geer R.E., Dekany I., Fendler J.H. Mechanism of and Defect Formation in the Self-Assembly of Polymeric Polycation-Montmorillonite Ultrathin Films. J. Am. Chem. Soc. 1997;119:6821–6832. doi: 10.1021/ja964409t. - DOI
-
- Zhang B., Shi S.X., Shi W.Y., Sun Z.Y., Kong X.G., Wei M., Duan X. Assembly of Ruthenium(II) Complex/Layered Double Hydroxide Ultrathin Film and Its Application as an Ultrasensitive Electrochemiluminescence Sensor. Electrochim. Acta. 2012;67:133–139. doi: 10.1016/j.electacta.2012.02.039. - DOI
-
- Caruso F., Schuler C. Enzyme Multilayers on Colloid Particles: Assembly, Stability, and Enzymatic Activity. Langmuir. 2000;16:9595–9603. doi: 10.1021/la000942h. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

