The Emerging Role of Cohesin in the DNA Damage Response
- PMID: 30487431
- PMCID: PMC6316000
- DOI: 10.3390/genes9120581
The Emerging Role of Cohesin in the DNA Damage Response
Abstract
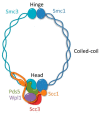
Faithful transmission of genetic material is crucial for all organisms since changes in genetic information may result in genomic instability that causes developmental disorders and cancers. Thus, understanding the mechanisms that preserve genome integrity is of fundamental importance. Cohesin is a multiprotein complex whose canonical function is to hold sister chromatids together from S-phase until the onset of anaphase to ensure the equal division of chromosomes. However, recent research points to a crucial function of cohesin in the DNA damage response (DDR). In this review, we summarize recent advances in the understanding of cohesin function in DNA damage signaling and repair. First, we focus on cohesin architecture and molecular mechanisms that govern sister chromatid cohesion. Next, we briefly characterize the main DDR pathways. Finally, we describe mechanisms that determine cohesin accumulation at DNA damage sites and discuss possible roles of cohesin in DDR.
Keywords: DNA damage tolerance; DNA double-strand breaks; cohesin; cohesin loader; replication stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

