Characterization of a Carbonyl Reductase from Rhodococcus erythropolis WZ010 and Its Variant Y54F for Asymmetric Synthesis of (S)- N-Boc-3-Hydroxypiperidine
- PMID: 30487432
- PMCID: PMC6321125
- DOI: 10.3390/molecules23123117
Characterization of a Carbonyl Reductase from Rhodococcus erythropolis WZ010 and Its Variant Y54F for Asymmetric Synthesis of (S)- N-Boc-3-Hydroxypiperidine
Abstract
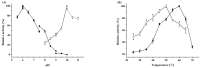
The recombinant carbonyl reductase from Rhodococcus erythropolis WZ010 (ReCR) demonstrated strict (S)-stereoselectivity and catalyzed the irreversible reduction of N-Boc-3-piperidone (NBPO) to (S)-N-Boc-3-hydroxypiperidine [(S)-NBHP], a key chiral intermediate in the synthesis of ibrutinib. The NAD(H)-specific enzyme was active within broad ranges of pH and temperature and had remarkable activity in the presence of higher concentration of organic solvents. The amino acid residue at position 54 was critical for the activity and the substitution of Tyr54 to Phe significantly enhanced the catalytic efficiency of ReCR. The kcat/Km values of ReCR Y54F for NBPO, (R/S)-2-octanol, and 2-propanol were 49.17 s-1 mM-1, 56.56 s-1 mM-1, and 20.69 s-1 mM-1, respectively. In addition, the (S)-NBHP yield was as high as 95.92% when whole cells of E. coli overexpressing ReCR variant Y54F catalyzed the asymmetric reduction of 1.5 M NBPO for 12 h in the aqueous/(R/S)-2-octanol biphasic system, demonstrating the great potential of ReCR variant Y54F for practical applications.
Keywords: (S)-N-Boc-3-hydroxypiperidine; Rhodococcus erythropolis; asymmetric reduction; carbonyl reductase; rational design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Characterization of a (2R,3R)-2,3-Butanediol Dehydrogenase from Rhodococcus erythropolis WZ010.Molecules. 2015 Apr 20;20(4):7156-73. doi: 10.3390/molecules20047156. Molecules. 2015. PMID: 25903366 Free PMC article.
-
Characterization of a stereospecific acetoin(diacetyl) reductase from Rhodococcus erythropolis WZ010 and its application for the synthesis of (2S,3S)-2,3-butanediol.Appl Microbiol Biotechnol. 2014 Jan;98(2):641-50. doi: 10.1007/s00253-013-4870-5. Epub 2013 Apr 9. Appl Microbiol Biotechnol. 2014. PMID: 23568047
-
Efficient synthesis of (S)-N-Boc-3-hydroxypiperidine using an (R)-specific carbonyl reductase from Candida parapsilosis.World J Microbiol Biotechnol. 2017 Mar;33(3):61. doi: 10.1007/s11274-016-2189-y. Epub 2017 Feb 27. World J Microbiol Biotechnol. 2017. PMID: 28243985
-
A kinetic study and application of a novel carbonyl reductase isolated from Rhodococcus erythropolis.Bioorg Med Chem. 1994 Jun;2(6):421-8. doi: 10.1016/0968-0896(94)80010-3. Bioorg Med Chem. 1994. PMID: 8000863
-
Efficient synthesis of Ibrutinib chiral intermediate in high space-time yield by recombinant E. coli co-expressing alcohol dehydrogenase and glucose dehydrogenase.RSC Adv. 2019 Jan 18;9(4):2325-2331. doi: 10.1039/c8ra08100j. eCollection 2019 Jan 14. RSC Adv. 2019. PMID: 35516114 Free PMC article. Review.
Cited by
-
Highly efficient synthesis of the chiral ACE inhibitor intermediate (R)-2-hydroxy-4-phenylbutyrate ethyl ester via engineered bi-enzyme coupled systems.Bioresour Bioprocess. 2024 Oct 15;11(1):99. doi: 10.1186/s40643-024-00814-z. Bioresour Bioprocess. 2024. PMID: 39402402 Free PMC article.
-
Efficient whole-cell oxidation of α,β-unsaturated alcohols to α,β-unsaturated aldehydes through the cascade biocatalysis of alcohol dehydrogenase, NADPH oxidase and hemoglobin.Microb Cell Fact. 2021 Jan 19;20(1):17. doi: 10.1186/s12934-021-01511-8. Microb Cell Fact. 2021. PMID: 33468136 Free PMC article.
-
Rhodococcus as A Versatile Biocatalyst in Organic Synthesis.Int J Mol Sci. 2019 Sep 26;20(19):4787. doi: 10.3390/ijms20194787. Int J Mol Sci. 2019. PMID: 31561555 Free PMC article. Review.
-
Engineering the Enantioselectivity of Yeast Old Yellow Enzyme OYE2y in Asymmetric Reduction of (E/Z)-Citral to (R)-Citronellal.Molecules. 2019 Mar 18;24(6):1057. doi: 10.3390/molecules24061057. Molecules. 2019. PMID: 30889828 Free PMC article.
-
Regioselective ring opening of aziridine for synthesizing azaheterocycle.Front Chem. 2023 Oct 19;11:1280633. doi: 10.3389/fchem.2023.1280633. eCollection 2023. Front Chem. 2023. PMID: 37927563 Free PMC article.
References
-
- Babu M.S., Raghunadh A., Ramulu K., Dahanukar V.H., Kumar U.K.S., Dubey P.K. A practical and enantiospecific synthesis of (−)-(R)- and (+)-(S)-piperidin-3-ols. Helv. Chim. Acta. 2014;97:1507–1515. doi: 10.1002/hlca.201400008. - DOI
-
- Chen L.-F., Zhang Y.-P., Fan H.-Y., Wu K., Lin J.-P., Wang H.-L., Wei D.-Z. Efficient bioreductive production of (R)-N-Boc-3-hydroxypiperidine by a carbonyl reductase. Catal. Commun. 2017;97:5–9. doi: 10.1016/j.catcom.2017.04.009. - DOI
-
- Ju X., Tang Y., Liang X., Hou M., Wan Z., Tao J. Development of a biocatalytic process to prepare (S)-N-Boc-3-hydroxypiperidine. Org. Process Res. Dev. 2014;18:827–830. doi: 10.1021/op500022y. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

