Chemoresistance and the Self-Maintaining Tumor Microenvironment
- PMID: 30487436
- PMCID: PMC6315745
- DOI: 10.3390/cancers10120471
Chemoresistance and the Self-Maintaining Tumor Microenvironment
Abstract
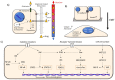
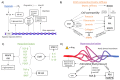
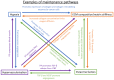
The progression of cancer is associated with alterations in the tumor microenvironment, including changes in extracellular matrix (ECM) composition, matrix rigidity, hypervascularization, hypoxia, and paracrine factors. One key malignant phenotype of cancer cells is their ability to resist chemotherapeutics, and elements of the ECM can promote chemoresistance in cancer cells through a variety of signaling pathways, inducing changes in gene expression and protein activity that allow resistance. Furthermore, the ECM is maintained as an environment that facilitates chemoresistance, since its constitution modulates the phenotype of cancer-associated cells, which themselves affect the microenvironment. In this review, we discuss how the properties of the tumor microenvironment promote chemoresistance in cancer cells, and the interplay between these external stimuli. We focus on both the response of cancer cells to the external environment, as well as the maintenance of the external environment, and how a chemoresistant phenotype emerges from the complex signaling network present.
Keywords: ECM; cancer stem cells; chemoresistance; fibrosis; hypervascularization; hypoxia; mechanosignaling; paracrine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Senthebane D.A., Rowe A., Thomford N.E., Shipanga H., Munro D., Mazeedi M., Almazyadi H.A.M., Kallmeyer K., Dandara C., Pepper M.S., et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int. J. Mol. Sci. 2017;18:1586. doi: 10.3390/ijms18071586. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

