Alston Virus, a Novel Paramyxovirus Isolated from Bats Causes Upper Respiratory Tract Infection in Experimentally Challenged Ferrets
- PMID: 30487438
- PMCID: PMC6315912
- DOI: 10.3390/v10120675
Alston Virus, a Novel Paramyxovirus Isolated from Bats Causes Upper Respiratory Tract Infection in Experimentally Challenged Ferrets
Erratum in
-
Erratum: Johnson et al. Alston Virus, a Novel Paramyxovirus Isolated from Bats Causes Upper Respiratory Tract Infection in Experimentally Challenged Ferrets. Viruses 2018, 10, 675.Viruses. 2021 Jun 23;13(7):1204. doi: 10.3390/v13071204. Viruses. 2021. PMID: 34201906 Free PMC article.
Abstract
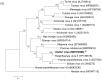
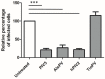
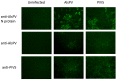
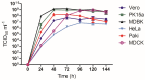
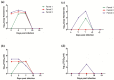
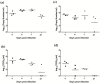
Multiple viruses with zoonotic potential have been isolated from bats globally. Here we describe the isolation and characterization of a novel paramyxovirus, Alston virus (AlsPV), isolated from urine collected from an Australian pteropid bat colony in Alstonville, New South Wales. Characterization of AlsPV by whole-genome sequencing and analyzing antigenic relatedness revealed it is a rubulavirus that is closely related to parainfluenza virus 5 (PIV5). Intranasal exposure of mice to AlsPV resulted in no clinical signs of disease, although viral RNA was detected in the olfactory bulbs of two mice at 21 days post exposure. Oronasal challenge of ferrets resulted in subclinical upper respiratory tract infection, viral shedding in respiratory secretions, and detection of viral antigen in the olfactory bulb of the brain. These results imply that AlsPV may be similar to PIV5 in its ability to infect multiple mammalian host species. This isolation of a novel paramyxovirus with the potential to transmit from bats to other mammalian species reinforces the importance of continued surveillance of bats as a source of emerging viruses.
Keywords: bat-borne; paramyxovirus; zoonoses.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Characterization of Teviot virus, an Australian bat-borne paramyxovirus.J Gen Virol. 2019 Mar;100(3):403-413. doi: 10.1099/jgv.0.001214. Epub 2019 Jan 28. J Gen Virol. 2019. PMID: 30688635
-
Evidence of bat origin for Menangle virus, a zoonotic paramyxovirus first isolated from diseased pigs.J Gen Virol. 2012 Dec;93(Pt 12):2590-2594. doi: 10.1099/vir.0.045385-0. Epub 2012 Aug 22. J Gen Virol. 2012. PMID: 22915696
-
Co-Circulation and Excretion Dynamics of Diverse Rubula- and Related Viruses in Egyptian Rousette Bats from South Africa.Viruses. 2019 Jan 8;11(1):37. doi: 10.3390/v11010037. Viruses. 2019. PMID: 30626055 Free PMC article.
-
Tools to study pathogen-host interactions in bats.Virus Res. 2018 Mar 15;248:5-12. doi: 10.1016/j.virusres.2018.02.013. Epub 2018 Feb 15. Virus Res. 2018. PMID: 29454637 Free PMC article. Review.
-
Public health awareness of emerging zoonotic viruses of bats: a European perspective.Vector Borne Zoonotic Dis. 2006 Winter;6(4):315-24. doi: 10.1089/vbz.2006.6.315. Vector Borne Zoonotic Dis. 2006. PMID: 17187565 Review.
Cited by
-
Genomic Characterization of a Relative of Mumps Virus in Lesser Dawn Bats of Southeast Asia.Viruses. 2023 Feb 28;15(3):659. doi: 10.3390/v15030659. Viruses. 2023. PMID: 36992368 Free PMC article.
-
A structure-based rationale for sialic acid independent host-cell entry of Sosuga virus.Proc Natl Acad Sci U S A. 2019 Oct 22;116(43):21514-21520. doi: 10.1073/pnas.1906717116. Epub 2019 Oct 7. Proc Natl Acad Sci U S A. 2019. PMID: 31591233 Free PMC article.
-
Eptesicus fuscus Orthorubulavirus, a Close Relative of Human Parainfluenza Virus 4, Discovered in a Bat in South Dakota.Microbiol Spectr. 2021 Oct 31;9(2):e0093021. doi: 10.1128/Spectrum.00930-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668744 Free PMC article.
-
Persistent paramyxovirus infections: in co-infections the parainfluenza virus type 5 persistent phenotype is dominant over the lytic phenotype.J Gen Virol. 2023 Nov;104(11):001916. doi: 10.1099/jgv.0.001916. J Gen Virol. 2023. PMID: 37962188 Free PMC article.
-
Viruses and Bats.Viruses. 2019 Sep 21;11(10):884. doi: 10.3390/v11100884. Viruses. 2019. PMID: 31546572 Free PMC article. No abstract available.
References
-
- Field H., Crameri G., Kung N.Y., Wang L.F. Ecological aspects of hendra virus. Curr. Top. Microbiol. Immunol. 2012;359:11–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

