Evaluation of Radiolabeled Girentuximab In Vitro and In Vivo
- PMID: 30487460
- PMCID: PMC6316122
- DOI: 10.3390/ph11040132
Evaluation of Radiolabeled Girentuximab In Vitro and In Vivo
Abstract
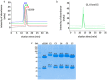
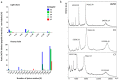
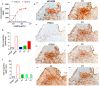
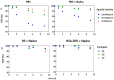
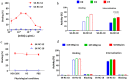
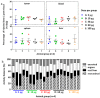
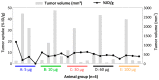
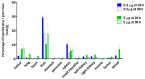
Girentuximab (cG250) targets carbonic anhydrase IX (CAIX), a protein which is expressed on the surface of most renal cancer cells (RCCs). cG250 labeled with 177Lu has been used in clinical trials for radioimmunotherapy (RIT) of RCCs. In this work, an extensive characterization of the immunoconjugates allowed optimization of the labeling conditions with 177Lu while maintaining immunoreactivity of cG250, which was then investigated in in vitro and in vivo experiments. cG250 was conjugated with S-2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (DOTA(SCN)) by using incubation times between 30 and 90 min and characterized by mass spectrometry. Immunoconjugates with five to ten DOTA(SCN) molecules per cG250 molecule were obtained. Conjugates with ratios less than six DOTA(SCN)/cG250 had higher in vitro antigen affinity, both pre- and postlabeling with 177Lu. Radiochemical stability increased, in the presence of sodium ascorbate, which prevents radiolysis. The immunoreactivity of the radiolabeled cG250 tested by specific binding to SK-RC-52 cells decreased when the DOTA content per conjugate increased. The in vivo tumor uptake was < 10% ID/g and independent of the total amount of protein in the range between 5 and 100 µg cG250 per animal. Low tumor uptake was found to be due to significant necrotic areas and heterogeneous CAIX expression. In addition, low vascularity indicated relatively poor accessibility of the CAIX target.
Keywords: 177Lu-radiopharmaceuticals; carbonic anhydrase IX; girentuximab; radioimmunotherapy; renal cell carcinomas.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Optimization of radioimmunotherapy of renal cell carcinoma: labeling of monoclonal antibody cG250 with 131I, 90Y, 177Lu, or 186Re.J Nucl Med. 2004 Feb;45(2):327-37. J Nucl Med. 2004. PMID: 14960657
-
[111In]-Labeled chimeric monoclonal antibody cG250 directed against carbonic anhydrase IX.2010 Aug 6 [updated 2010 Sep 2]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Aug 6 [updated 2010 Sep 2]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20827818 Free Books & Documents. Review.
-
Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma.Eur Urol. 2013 Sep;64(3):478-85. doi: 10.1016/j.eururo.2012.08.024. Epub 2012 Aug 21. Eur Urol. 2013. PMID: 22980441 Clinical Trial.
-
Optical Imaging of Renal Cell Carcinoma with Anti-Carbonic Anhydrase IX Monoclonal Antibody Girentuximab.J Nucl Med. 2014 Jun;55(6):1035-40. doi: 10.2967/jnumed.114.137356. Epub 2014 Apr 21. J Nucl Med. 2014. PMID: 24752673
-
Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy.Eur Urol. 2010 Jul;58(1):75-83. doi: 10.1016/j.eururo.2010.03.015. Epub 2010 Mar 23. Eur Urol. 2010. PMID: 20359812 Review.
Cited by
-
Comparison of carbonic anhydrase-IX-targeted trifunctional radioligands between linear- and branched-chain arrangements.Front Nucl Med. 2025 Apr 16;5:1585027. doi: 10.3389/fnume.2025.1585027. eCollection 2025. Front Nucl Med. 2025. PMID: 40308720 Free PMC article.
-
Antibody diversity in IVIG: Therapeutic opportunities for novel immunotherapeutic drugs.Front Immunol. 2023 Mar 28;14:1166821. doi: 10.3389/fimmu.2023.1166821. eCollection 2023. Front Immunol. 2023. PMID: 37063852 Free PMC article. Review.
-
Isolation and characterization of monoclonal antibodies against human carbonic anhydrase-IX.MAbs. 2021 Jan-Dec;13(1):1999194. doi: 10.1080/19420862.2021.1999194. MAbs. 2021. PMID: 34806527 Free PMC article.
References
-
- Brouwers A.H., van Eerd J.E., Frielink C., Oosterwijk E., Oyen W.J., Corstens F.H., Boerman O.C. Optimization of radioimmunotherapy of renal cell carcinoma: Labeling of monoclonal antibody cG250 with 131I, 90Y, 177Lu, or 186Re. J. Nucl. Med. 2004;45:327–337. - PubMed
-
- Thakral P., Singla S., Yadav M.P., Vasisht A., Sharma A., Gupta S.K., Bal C.S., Malhotra A. An approach for conjugation of (177) Lu-DOTA-SCN-Rituximab (BioSim) & its evaluation for radioimmunotherapy of relapsed & refractory B-cell non Hodgkins lymphoma patients. Indian J. Med. Res. 2014;139:544–554. - PMC - PubMed
-
- Zalutsky M.R., Reardon D.A., Akabani G., Coleman R.E., Friedman A.H., Friedman H.S., McLendon R.E., Wong T.Z., Bigner D.D. Clinical experience with alpha-particle emitting 211At: Treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J. Nucl. Med. 2008;49:30–38. doi: 10.2967/jnumed.107.046938. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

