Heme oxygenase-1 prevents murine intestinal inflammation
- PMID: 30487665
- PMCID: PMC6252298
- DOI: 10.3164/jcbn.17-133
Heme oxygenase-1 prevents murine intestinal inflammation
Abstract
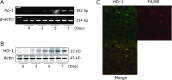
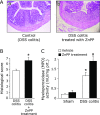
Heme oxygenases (HOs) are rate-limiting enzymes catabolizing heme to biliverdin, ferrous iron, and carbon monoxide, and of the three HO isoforms identified, HO-1 plays a protective role against inflammatory processes. In this study, we investigated the possible role of HO-1 in intestinal inflammation. Acute colitis was induced in male C57BL/6 (wild-type) and homozygous BTB and CNC homolog 1 (Bach1)-deficient mice, which show high HO-1 expression in the colonic mucosa, using dextran sodium sulfate. The disease activity index, myeloperoxidase activity, and inflammatory cytokines in the colonic mucosa were evaluated 7 days after dextran sodium sulfate-dependent colitis induction. We also evaluated the impact of HO-1 inhibition using zinc protoporphyrin IX (25 mg/kg i.p., daily). After dextran sodium sulfate administration, HO-1 mRNA and protein expression increased in a time-dependent manner. Disease activity index score, myeloperoxidase activity, and colonic production of TNF-α and IFN-γ were increased after dextran sodium sulfate administration, and co-administration of zinc protoporphyrin IX enhanced their increase. In addition, disease activity index in Bach1-deficient was significantly lower after dextran sodium sulfate administration than that in wild type mice. These results indicate that HO-1 plays a protective role against dextran sodium sulfate-induced intestinal inflammation, possibly by regulating pro-inflammatory cytokines in intestinal tissues.
Keywords: BTB and CNC homolog 1 (Bach1); dextran sodium sulfate (DSS)-induced colitis; heme oxygenase-1 (HO-1).
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures




References
-
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321. - PubMed
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
-
- Takagi T, Naito Y, Yoshikawa T. Free radicals in inflammatory bowel disease. In: Naito Y, Suematsu M, Yoshikawa T, editors. Frontiers of Gastrointestinal Research; Free Radical Biology in Digestive Disease. Basel (Switzerland): Karger Publishers; 2011. pp. 128–136.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

