High phosphate diet suppresses lipogenesis in white adipose tissue
- PMID: 30487667
- PMCID: PMC6252294
- DOI: 10.3164/jcbn.17-141
High phosphate diet suppresses lipogenesis in white adipose tissue
Abstract
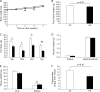
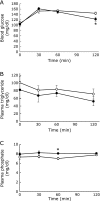
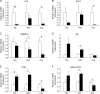
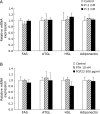
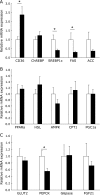
Excessive phosphate intake has been positively associated with renal and vascular dysfunction, conversely negatively associated with body fat accumulation. We investigated the effect of a high-phosphate diet on the expression of lipid metabolic genes in white adipose tissue and liver. Male 8-week-old Sprague-Dawley rats were fed a control diet containing 0.6% phosphate or a high-phosphate diet containing 1.5% phosphate for 4 weeks. In comparison to the control group, the HP group showed a significantly lower body fat mass and fasting plasma insulin level alongside decreased lipogenic and increased lipolytic gene expression in visceral fat. Additionally, the expression of genes involved in hepatic lipogenesis, hepatic glycogenesis, and triglyceride accumulation decreased in the high-phosphate group. Exogenous phosphate, parathyroid hormone, and fibroblast growth factor 23 did not directly affect the expression of lipolytic or lipogenic genes in 3T3-L1 adipocytes and HepG2 hepatocytes. Thus, the high-phosphate diet suppressed the activity of white adipose tissue by increasing lipolytic gene expression and decreasing lipogenic gene expression. These effects could have been caused by the lowered fasting plasma insulin level that occurred in response to the high-phosphate diet, but were not directly caused by the increases in plasma phosphate or phosphaturic hormones.
Keywords: dietary phosphate; insulin; lipogenesis; lipolysis; white adipose tissue.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures



References
-
- Berndt TJ, Schiavi S, Kumar R. “Phosphatonins” and the regulation of phosphorus homeostasis. Am J Physiol Ren Physiol. 2005;289:F1170–F1182. - PubMed
-
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. - PubMed
-
- Fujii H, Joki N. Mineral metabolism and cardiovascular disease in CKD. Clin Exp Nephrol. 2017;21 (Suppl 1):53–63. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

