Distinct Microglial Responses in Two Transgenic Murine Models of TAU Pathology
- PMID: 30487735
- PMCID: PMC6246744
- DOI: 10.3389/fncel.2018.00421
Distinct Microglial Responses in Two Transgenic Murine Models of TAU Pathology
Abstract
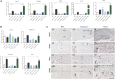
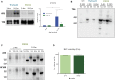
Microglial cells are crucial players in the pathological process of neurodegenerative diseases, such as Alzheimer's disease (AD). Microglial response in AD has been principally studied in relation to amyloid-beta pathology but, comparatively, little is known about inflammatory processes associated to tau pathology. In the hippocampus of AD patients, where tau pathology is more prominent than amyloid-beta pathology, a microglial degenerative process has been reported. In this work, we have directly compared the microglial response in two different transgenic tau mouse models: ThyTau22 and P301S. Surprisingly, these two models showed important differences in the microglial profile and tau pathology. Where ThyTau22 hippocampus manifested mild microglial activation, P301S mice exhibited a strong microglial response in parallel with high phospho-tau accumulation. This differential phospho-tau expression could account for the different microglial response in these two tau strains. However, soluble (S1) fractions from ThyTau22 hippocampus presented relatively high content of soluble phospho-tau (AT8-positive) and were highly toxic for microglial cells in vitro, whereas the correspondent S1 fractions from P301S mice displayed low soluble phospho-tau levels and were not toxic for microglial cells. Therefore, not only the expression levels but the aggregation of phospho-tau should differ between both models. In fact, most of tau forms in the P301S mice were aggregated and, in consequence, forming insoluble tau species. We conclude that different factors as tau mutations, accumulation, phosphorylation, and/or aggregation could account for the distinct microglial responses observed in these two tau models. For this reason, deciphering the molecular nature of toxic tau species for microglial cells might be a promising therapeutic approach in order to restore the deficient immunological protection observed in AD hippocampus.
Keywords: Alzheimer disease; inflammation; microglia; tau models; tauopathies.
Figures