Polymorphisms in mTOR and Calcineurin Signaling Pathways Are Associated With Long-Term Clinical Outcomes in Kidney Transplant Recipients
- PMID: 30487748
- PMCID: PMC6246626
- DOI: 10.3389/fphar.2018.01296
Polymorphisms in mTOR and Calcineurin Signaling Pathways Are Associated With Long-Term Clinical Outcomes in Kidney Transplant Recipients
Abstract
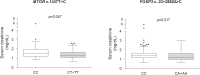
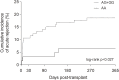
Monitoring of immunosuppressive drugs, such as calcineurin and mTOR inhibitors, is essential to avoid undesirable kidney transplant outcomes. Polymorphisms in pharmacokinetics-related genes have been associated with variability in blood levels of immunosuppressive drugs and adverse effects, but influence of pharmacodynamics-related genes remains to be elucidated. The influence of polymorphisms in genes of the mTOR and calcineurin signaling pathways on long-term clinical outcomes was investigated in Brazilian kidney transplant recipients within the 1-year post-transplant. Two-hundred and sixty-nine kidney transplant recipients were enrolled at a kidney transplant center in São Paulo city, Brazil, and treated with tacrolimus plus everolimus or mycophenolate sodium (clinical trial NCT01354301). Clinical and laboratory data, including renal function parameters and drug blood levels were recorded. Genomic DNA was extracted from blood samples. Polymorphisms in MTOR rs1057079 (c.4731G>A), rs1135172 (c.1437T>C), and rs1064261 (c.2997C>T); PPP3CA rs3730251 (c.249G>A); FKBP1A rs6033557 (n.259+24936T>C); FKBP2 rs2159370 (c.-2110G>T); and FOXP3 rs3761548 (c.-23+2882A>C) and rs2232365 (c.-22-902A>G) were analyzed by real-time PCR. Frequencies of gene polymorphisms did not differ among the treatment groups. Analysis of primary outcomes showed that patients carrying MTOR c.1437CC and FOXP3 c.-23+2882CC genotypes had higher serum creatinine than non-carriers (p < 0.05) at 1-year post-transplant. MTOR c.4731G allele (AG+GG genotype) was associated with increased risk for acute rejection (OR = 3.53, 95% CI = 1.09-11.48, p = 0.037). Moreover, 1-year cumulative incidence of rejection was higher in MTOR c.4731G allele carriers compared to AA genotype carriers (p = 0.027). Individually, analysis of secondary outcomes revealed that FKBP2 c.-2110GG genotype carriers had higher risk of leukopenia, FKBP1A n.259+24936C allele carriers had increased risk of constipation, and FOXP3 c.-22-902A or c.-23+2882A allele had higher risk of gastrointestinal disorders (p < 0.05). However, these results were not maintained in the multivariable analysis after p-value adjustment. In conclusion, variants in genes of mTOR and calcineurin pathways are associated with long-term impaired renal function, increased risk of acute rejection, and, individually, with adverse events in Brazilian kidney transplant recipients.
Keywords: FOXP3; calcineurin; immunosuppressive drugs; kidney transplant; mTOR; pharmacogenetics.
Figures
Similar articles
-
Differential expression of genes related to calcineurin and mTOR signaling and regulatory miRNAs in peripheral blood from kidney recipients under tacrolimus-based therapy.Ann Transl Med. 2020 Sep;8(17):1051. doi: 10.21037/atm-20-1757. Ann Transl Med. 2020. PMID: 33145270 Free PMC article.
-
Association of the PPP3CA c.249G>A variant with clinical outcomes of tacrolimus-based therapy in kidney transplant recipients.Pharmgenomics Pers Med. 2017 Mar 31;10:101-106. doi: 10.2147/PGPM.S131390. eCollection 2017. Pharmgenomics Pers Med. 2017. PMID: 28435308 Free PMC article.
-
Impact of FOXP3 Polymorphisms on the Blood Level of Tacrolimus in Renal Transplant Recipients.Transplant Proc. 2016 Jul-Aug;48(6):1962-7. doi: 10.1016/j.transproceed.2016.04.016. Transplant Proc. 2016. PMID: 27569929
-
Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II.Clin Pharmacokinet. 2010 Apr;49(4):207-21. doi: 10.2165/11317550-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20214406 Review.
-
Treatment strategies in pediatric solid organ transplant recipients with calcineurin inhibitor-induced nephrotoxicity.Pediatr Transplant. 2006 Sep;10(6):721-9. doi: 10.1111/j.1399-3046.2006.00577.x. Pediatr Transplant. 2006. PMID: 16911497 Review.
Cited by
-
Differential expression of genes related to calcineurin and mTOR signaling and regulatory miRNAs in peripheral blood from kidney recipients under tacrolimus-based therapy.Ann Transl Med. 2020 Sep;8(17):1051. doi: 10.21037/atm-20-1757. Ann Transl Med. 2020. PMID: 33145270 Free PMC article.
-
Proteome-wide Mendelian randomization reveals the causal effects of immune-related plasma proteins on psychiatric disorders.Hum Genet. 2023 Jun;142(6):809-818. doi: 10.1007/s00439-023-02562-0. Epub 2023 Apr 21. Hum Genet. 2023. PMID: 37085628
-
Influence of genetic polymorphisms on pharmacokinetics and treatment response of mycophenolic acid: a scoping review.Pharmacogenomics. 2024;25(5-6):259-288. doi: 10.1080/14622416.2024.2344430. Epub 2024 May 16. Pharmacogenomics. 2024. PMID: 38884938 Free PMC article.
References
-
- de Paula M. I., Medina Pestana J. O., Nicolau Ferreira A., Pontello Cristelli M., Fabiano Franco M., Aguiar W. F., et al. (2016). Long-term follow-up of de novo use of mTOR and calcineurin inhibitors after kidney transplantation. Ther. Drug Monit. 38 22–31. 10.1097/FTD.0000000000000227 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

