Microbial Biomarkers of Intestinal Barrier Maturation in Preterm Infants
- PMID: 30487786
- PMCID: PMC6246636
- DOI: 10.3389/fmicb.2018.02755
Microbial Biomarkers of Intestinal Barrier Maturation in Preterm Infants
Abstract
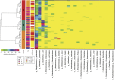
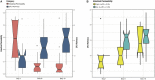
Intestinal barrier immaturity, or "leaky gut," is the proximate cause of susceptibility to necrotizing enterocolitis in preterm neonates. However, the impact of intestinal microbiota development on intestinal mucosal barrier maturation has not been evaluated in this population. In this study, we investigated a longitudinally sampled cohort of 38 preterm infants < 33 weeks gestation monitored for intestinal permeability (IP) and fecal microbiota during the first 2 weeks of life. Rapid decrease in IP indicating intestinal barrier function maturation correlated with significant increase in community diversity. In particular, members of the Clostridiales and Bifidobacterium were highly transcriptionally active, and progressively increasing abundance in Clostridiales was significantly associated with decreased intestinal permeability. Further, neonatal factors previously identified to promote intestinal barrier maturation, including early exclusive breastmilk feeding and shorter duration antibiotic exposure, associate with the early colonization of the intestinal microbiota by members of the Clostridiales, which altogether are associated with improved intestinal barrier function in preterm infants.
Keywords: Bifidobacterium; Clostridiales; breastmilk feeding; intestinal microbiota; intestinal permeability; leaky gut; necrotizing enterocolitis; preterm infant.
Figures


References
-
- Albers M. J., Steyerberg E. W., Hazebroek F. W., Mourik M., Borsboom G. J., Rietveld T., et al. (2005). Glutamine supplementation of parenteral nutrition does not improve intestinal permeability, nitrogen balance, or outcome in newborns and infants undergoing digestive-tract surgery: results from a double-blind, randomized, controlled trial. Ann. Surg. 241 599–606. 10.1097/01.sla.0000157270.24991.71 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

