Effect of Age on NK Cell Compartment in Chronic Myeloid Leukemia Patients Treated With Tyrosine Kinase Inhibitors
- PMID: 30487792
- PMCID: PMC6246921
- DOI: 10.3389/fimmu.2018.02587
Effect of Age on NK Cell Compartment in Chronic Myeloid Leukemia Patients Treated With Tyrosine Kinase Inhibitors
Abstract
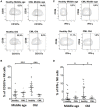
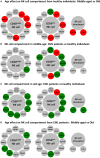
Natural killer (NK) cells are a very important component of the innate immune response involved in the lysis of virus infected and tumor cells. Aging has a profound impact in the frequency, phenotype and function of NK cells. Chronic Myeloid Leukemia (CML) is caused by the BCR-ABL gene formation encoding aberrant oncoprotein tyrosine kinase. Treatment with tyrosine kinase inhibitors (TKIs) induces durable deep molecular response. The response to treatment and life expectancy is lower in older patients with chronic phase of CML than in younger patients. In this work we analyse NK cells from TKI-treated CML patients and healthy controls stratified according to age. We have analyzed the expression of NK receptors, activation markers, NK cell differentiation in CD56bright and CD56dim NK cell subsets and the expression of CD107a and IFN-γ in NK cells stimulated with K562. Whereas significant differences on the phenotype and function of NK cells were found between middle-aged (35-65 years old) and elderly (older than 65) healthy individuals, NK cells from TKI-treated CML patients do not show significant differences related with age in most parameters studied, indicating that age is not a limitation of the NK cell recovery after treatment with TKI. Our results also revealed differences in the expression of NK receptors, activation markers and functional assays in NK cells from TKI-treated CML patients compared with age-matched healthy controls. These results highlight the relevance of NK cells in TKI-treated patients and the need of an extensive analysis of the effect of aging on NK cell phenotype and function in these patients in order to define new NK-cell based strategies directed to control CML progression and achieve long-term disease remission after TKI cessation.
Keywords: CML; NK cell subsets; NK receptors; activation markers; aging; cytokines; differentiation markers; tyrosine kinase inhibitors.
Figures




References
-
- Tarazona R, Casado JG, DelaRosa O, Torre-Cisneros J, Villanueva JL, Sanchez B, et al. . Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol. (2002) 22:176–83. 10.1023/A:1015476114409 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

