Impaired COMMD10-Mediated Regulation of Ly6Chi Monocyte-Driven Inflammation Disrupts Gut Barrier Function
- PMID: 30487795
- PMCID: PMC6246736
- DOI: 10.3389/fimmu.2018.02623
Impaired COMMD10-Mediated Regulation of Ly6Chi Monocyte-Driven Inflammation Disrupts Gut Barrier Function
Abstract
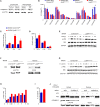
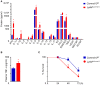
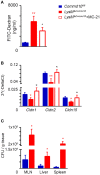
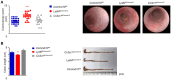
Ly6Chi monocyte tissue infiltrates play important roles in mediating local inflammation, bacterial elimination and resolution during sepsis and inflammatory bowel disease (IBD). Yet, the immunoregulatory pathways dictating their activity remain poorly understood. COMMD family proteins are emerging as key regulators of signaling and protein trafficking events during inflammation, but the specific role of COMMD10 in governing Ly6Chi monocyte-driven inflammation is unknown. Here we report that COMMD10 curbs canonical and non-canonical inflammasome activity in Ly6Chi monocytes in a model of LPS-induced systemic inflammation. Accordingly, its deficiency in myeloid cells, but not in tissue resident macrophages, resulted in increased Ly6Chi monocyte liver and colonic infiltrates, elevated systemic cytokine storm, increased activation of caspase-1 and-11 in the liver and colon, and augmented IL-1β production systemically and specifically in LPS-challenged circulating Ly6Chi monocytes. These inflammatory manifestations were accompanied by impaired intestinal barrier function with ensuing bacterial dissemination to the mesenteric lymph nodes and liver leading to increased mortality. The increased inflammasome activity and intestinal barrier leakage were ameliorated by the inducible ablation of COMMD10-deficient Ly6Chi monocytes. In consistence with these results, COMMD10-deficiency in Ly6Chi monocytes, but not in intestinal-resident lamina propria macrophages, led to increased IL-1β production and aggravated colonic inflammation in a model of DSS-induced colitis. Finally, COMMD10 expression was reduced in Ly6Chi monocytes and their corresponding human CD14hi monocytes sorted from mice subjected to DSS-induced colitis or from IBD patients, respectively. Collectively, these results highlight COMMD10 as a negative regulator of Ly6Chi monocyte inflammasome activity during systemic inflammation and IBD.
Keywords: COMMD10; Ly6Chi monocytes; colitis; inflammasome; systemic inflammation.
Figures


Similar articles
-
COMMD10 is critical for Kupffer cell survival and controls Ly6Chi monocyte differentiation and inflammation in the injured liver.Cell Rep. 2021 Nov 16;37(7):110026. doi: 10.1016/j.celrep.2021.110026. Cell Rep. 2021. PMID: 34788631 Free PMC article.
-
Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6C(high) CCR2(+) inflammatory monocyte/macrophage-derived CCL11.J Immunol. 2011 May 15;186(10):5993-6003. doi: 10.4049/jimmunol.1003844. Epub 2011 Apr 15. J Immunol. 2011. PMID: 21498668 Free PMC article.
-
Dynamics of Colon Monocyte and Macrophage Activation During Colitis.Front Immunol. 2018 Nov 27;9:2764. doi: 10.3389/fimmu.2018.02764. eCollection 2018. Front Immunol. 2018. PMID: 30542349 Free PMC article.
-
Biodegradable microspheres targeting mucosal immune-regulating cells: new approach for treatment of inflammatory bowel disease.J Gastroenterol. 2003 Mar;38 Suppl 15:59-62. J Gastroenterol. 2003. PMID: 12698874 Review.
-
Dynamic role of macrophage CX3CR1 expression in inflammatory bowel disease.Immunol Lett. 2021 Apr;232:39-44. doi: 10.1016/j.imlet.2021.02.001. Epub 2021 Feb 11. Immunol Lett. 2021. PMID: 33582183 Review.
Cited by
-
COMMD10-Guided Phagolysosomal Maturation Promotes Clearance of Staphylococcus aureus in Macrophages.iScience. 2019 Apr 26;14:147-163. doi: 10.1016/j.isci.2019.03.024. Epub 2019 Mar 27. iScience. 2019. PMID: 30959277 Free PMC article.
-
Inflammasome Regulation: Therapeutic Potential for Inflammatory Bowel Disease.Molecules. 2021 Mar 19;26(6):1725. doi: 10.3390/molecules26061725. Molecules. 2021. PMID: 33808793 Free PMC article. Review.
-
COMMD10 is critical for Kupffer cell survival and controls Ly6Chi monocyte differentiation and inflammation in the injured liver.Cell Rep. 2021 Nov 16;37(7):110026. doi: 10.1016/j.celrep.2021.110026. Cell Rep. 2021. PMID: 34788631 Free PMC article.
-
COMMD10 Is Essential for Neural Plate Development during Embryogenesis.J Dev Biol. 2023 Mar 16;11(1):13. doi: 10.3390/jdb11010013. J Dev Biol. 2023. PMID: 36976102 Free PMC article.
-
Commander Complex-A Multifaceted Operator in Intracellular Signaling and Cargo.Cells. 2021 Dec 7;10(12):3447. doi: 10.3390/cells10123447. Cells. 2021. PMID: 34943955 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

