Laparoscopic and endoscopic cooperative surgery for gastric tumors: Perspective for actual practice and oncological benefits
- PMID: 30487950
- PMCID: PMC6247108
- DOI: 10.4251/wjgo.v10.i11.381
Laparoscopic and endoscopic cooperative surgery for gastric tumors: Perspective for actual practice and oncological benefits
Abstract
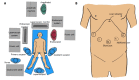
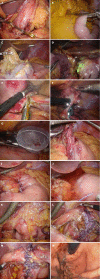
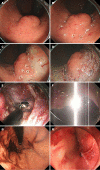
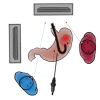
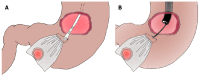
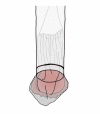
Laparoscopic and endoscopic cooperative surgery (LECS) is a surgical technique that combines laparoscopic partial gastrectomy and endoscopic submucosal dissection. LECS requires close collaboration between skilled laparoscopic surgeons and experienced endoscopists. For successful LECS, experience alone is not sufficient. Instead, familiarity with the characteristics of both laparoscopic surgery and endoscopic intervention is necessary to overcome various technical problems. LECS was developed mainly as a treatment for gastric submucosal tumors without epithelial lesions, including gastrointestinal stromal tumors (GISTs). Local gastric wall dissection without lymphadenectomy is adequate for the treatment of gastric GISTs. Compared with conventional simple wedge resection with a linear stapler, LECS can provide both optimal surgical margins and oncological benefit that result in functional preservation of the residual stomach. As technical characteristics, however, classic LECS involves intentional opening of the gastric wall, resulting in a risk of tumor dissemination with contamination by gastric juice. Therefore, several modified LECS techniques have been developed to avoid even subtle tumor exposure. Furthermore, LECS for early gastric cancer has been attempted according to the concept of sentinel lymph node dissection. LECS is a prospective treatment for GISTs and might become a future therapeutic option even for early gastric cancer. Interventional endoscopists and laparoscopic surgeons collaboratively explore curative resection. Simultaneous intraluminal approach with endoscopy allows surgeons to optimizes the resection area. LECS, not simple wedge resection, achieves minimally invasive treatment and allows for oncologically precise resection. We herein present detailed tips and pitfalls of LECS and discuss various technical considerations.
Keywords: Early gastric cancer; Facility-based; Gastrointestinal stromal tumor; Laparoscopic and endoscopic cooperative surgery; Minimally invasive surgery.
Conflict of interest statement
Conflict-of-interest statement: No author has potential conflicts of interest.
Figures


Similar articles
-
Laparoscopic endoscopic cooperative surgery as a minimally invasive treatment for gastric submucosal tumor.World J Gastrointest Endosc. 2015 Oct 10;7(14):1150-6. doi: 10.4253/wjge.v7.i14.1150. World J Gastrointest Endosc. 2015. PMID: 26468339 Free PMC article.
-
Laparoscopic and endoscopic cooperative surgery is a feasible treatment procedure for intraluminal gastric gastrointestinal stromal tumors compared to endoscopic intragastric surgery.Surg Endosc. 2018 Jan;32(1):351-357. doi: 10.1007/s00464-017-5683-x. Epub 2017 Jun 29. Surg Endosc. 2018. PMID: 28664426
-
Laparoscopic endoscopic cooperative surgery (LECS) for the upper gastrointestinal tract.Transl Gastroenterol Hepatol. 2017 May 5;2:40. doi: 10.21037/tgh.2017.03.20. eCollection 2017. Transl Gastroenterol Hepatol. 2017. PMID: 28616596 Free PMC article. Review.
-
Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications.Ann Gastroenterol Surg. 2019 Feb 19;3(3):239-246. doi: 10.1002/ags3.12238. eCollection 2019 May. Ann Gastroenterol Surg. 2019. PMID: 31131352 Free PMC article. Review.
-
Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor.Gastric Cancer. 2017 May;20(3):553-557. doi: 10.1007/s10120-016-0641-1. Epub 2016 Sep 6. Gastric Cancer. 2017. PMID: 27599829 Clinical Trial.
Cited by
-
Early gastric cancer: A challenge in Western countries.World J Gastroenterol. 2022 Feb 21;28(7):693-703. doi: 10.3748/wjg.v28.i7.693. World J Gastroenterol. 2022. PMID: 35317273 Free PMC article.
-
Non-exposed endoscopic wall-inversion surgery with one-step nucleic acid amplification for early gastrointestinal tumors: Personal experience and literature review.World J Gastroenterol. 2023 Jun 28;29(24):3883-3898. doi: 10.3748/wjg.v29.i24.3883. World J Gastroenterol. 2023. PMID: 37426319 Free PMC article. Review.
-
Burgeoning study of sentinel-node analysis on management of early gastric cancer after endoscopic submucosal dissection.World J Gastrointest Endosc. 2020 Apr 16;12(4):119-127. doi: 10.4253/wjge.v12.i4.119. World J Gastrointest Endosc. 2020. PMID: 32341748 Free PMC article. Review.
-
Postoperative hypotension in gastric cancer patients: causes and clinical approaches.Support Care Cancer. 2025 Aug 7;33(9):763. doi: 10.1007/s00520-025-09834-7. Support Care Cancer. 2025. PMID: 40775538 Review.
-
Gastrointestinal Stromal Tumors (GISTs) in Pediatric Patients: A Case Report and Literature Review.Children (Basel). 2024 Aug 26;11(9):1040. doi: 10.3390/children11091040. Children (Basel). 2024. PMID: 39334573 Free PMC article. Review.
References
-
- Liang H, Liang W, Lei Z, Liu Z, Wang W, He J, Zeng Y, Huang W, Wang M, Chen Y, et al. Three-Dimensional Versus Two-Dimensional Video-Assisted Endoscopic Surgery: A Meta-analysis of Clinical Data. World J Surg. 2018 Epub ahead of print. - PubMed
-
- Abe N, Takeuchi H, Ohki A, Hashimoto Y, Mori T, Sugiyama M. Comparison between endoscopic and laparoscopic removal of gastric submucosal tumor. Dig Endosc. 2018;30 Suppl 1:7–16. - PubMed
-
- Yin X, Yin Y, Chen H, Shen C, Tang S, Cai Z, Zhang B, Chen Z. Comparison Analysis of Three Different Types of Minimally Invasive Procedures for Gastrointestinal Stromal Tumors ≤5 cm. J Laparoendosc Adv Surg Tech A. 2018;28:58–64. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

