Multiple biomarker panel to screen for severe aortic stenosis: results from the CASABLANCA study
- PMID: 30487984
- PMCID: PMC6242008
- DOI: 10.1136/openhrt-2018-000916
Multiple biomarker panel to screen for severe aortic stenosis: results from the CASABLANCA study
Abstract
Objective: Severe aortic valve stenosis (AS) develops via insidious processes and can be challenging to correctly diagnose. We sought to develop a circulating biomarker panel to identify patients with severe AS.
Methods: We enrolled study participants undergoing coronary or peripheral angiography for a variety of cardiovascular diseases at a single academic medical centre. A panel of 109 proteins were measured in blood obtained at the time of the procedure. Statistical learning methods were used to identify biomarkers and clinical parameters that associate with severe AS. A diagnostic model incorporating clinical and biomarker results was developed and evaluated using Monte Carlo cross-validation.
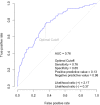
Results: Of 1244 subjects (age 66.4±11.5 years, 28.7% female), 80 (6.4%) had severe AS (defined as aortic valve area (AVA) <1.0 cm2). A final model included age, N-terminal pro-B-type natriuretic peptide, von Willebrand factor and fetuin-A. The model had good discrimination for severe AS (OR=5.9, 95% CI 3.5 to 10.1, p<0.001) with an area under the curve of 0.76 insample and 0.74 with cross-validation. A diagnostic score was generated. Higher prevalence of severe AS was noted in those with higher scores, such that 1.6% of those with a score of 1 had severe AS compared with 15.3% with a score of 5 (p<0.001), and score values were inversely correlated with AVA (r=-0.35; p<0.001). At optimal model cut-off, we found 76% sensitivity, 65% specificity, 13% positive predictive value and 98% negative predictive value.
Conclusions: We describe a novel, multiple biomarker approach for diagnostic evaluation of severe AS.
Trial registration number: NCT00842868.
Keywords: aortic stenosis; biomarkers; valvular heart disease.
Conflict of interest statement
Competing interests: SE receives institutional research support from Siemens and Boehringer Ingelheim Pharmaceuticals and consulting fees from Medtronic and Edwards Lifesciences. The remaining authors have no relationships to disclose. JLJ has received grant support from Abbott Diagnostics, Roche Diagnostics, Singulex and Prevencio; consulting income from Roche Diagnostics, Critical Diagnostics, Philips and Novartis; and participates in clinical endpoint committees/data safety monitoring boards for Novartis, Amgen, AbbVie, Pfizer, Janssen and Boehringer Ingelheim. CM is a consultant to Prevencio. RR and GB are employees of Prevencio. Siemens Diagnostics had no involvement in the present study design, collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. All other authors have reported that they have no relationships relevant to the content of this paper to disclose.
Figures


References
-
- Nishimura RA, Otto CM, Bonow RO, et al. . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–185. 10.1016/j.jacc.2014.02.536 - DOI - PubMed
-
- Elmariah S, Palacios IF, McAndrew T, et al. . Outcomes of transcatheter and surgical aortic valve replacement in high-risk patients with aortic stenosis and left ventricular dysfunction: results from the Placement of Aortic Transcatheter Valves (PARTNER) trial (cohort A). Circ Cardiovasc Interv 2013;6:604–14. 10.1161/CIRCINTERVENTIONS.113.000650 - DOI - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
