Current state of Alzheimer's fluid biomarkers
- PMID: 30488277
- PMCID: PMC6280827
- DOI: 10.1007/s00401-018-1932-x
Current state of Alzheimer's fluid biomarkers
Abstract
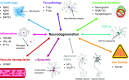
Alzheimer's disease (AD) is a progressive neurodegenerative disease with a complex and heterogeneous pathophysiology. The number of people living with AD is predicted to increase; however, there are no disease-modifying therapies currently available and none have been successful in late-stage clinical trials. Fluid biomarkers measured in cerebrospinal fluid (CSF) or blood hold promise for enabling more effective drug development and establishing a more personalized medicine approach for AD diagnosis and treatment. Biomarkers used in drug development programmes should be qualified for a specific context of use (COU). These COUs include, but are not limited to, subject/patient selection, assessment of disease state and/or prognosis, assessment of mechanism of action, dose optimization, drug response monitoring, efficacy maximization, and toxicity/adverse reactions identification and minimization. The core AD CSF biomarkers Aβ42, t-tau, and p-tau are recognized by research guidelines for their diagnostic utility and are being considered for qualification for subject selection in clinical trials. However, there is a need to better understand their potential for other COUs, as well as identify additional fluid biomarkers reflecting other aspects of AD pathophysiology. Several novel fluid biomarkers have been proposed, but their role in AD pathology and their use as AD biomarkers have yet to be validated. In this review, we summarize some of the pathological mechanisms implicated in the sporadic AD and highlight the data for several established and novel fluid biomarkers (including BACE1, TREM2, YKL-40, IP-10, neurogranin, SNAP-25, synaptotagmin, α-synuclein, TDP-43, ferritin, VILIP-1, and NF-L) associated with each mechanism. We discuss the potential COUs for each biomarker.
Keywords: Alzheimer’s disease; Amyloid; Biomarker; Blood; Cerebrospinal fluid; Tau.
Conflict of interest statement
JLM is Principal Investigator of trials funded by Roche, Merck, Novartis, and Janssen and has received speaker or consultant fees from Roche, Roche Diagnostics, Biogen, Merck, Novartis, Oryzon, IBL, Axovant, Lundbeck, and Lilly. SA receives current research support from the National Health and Medical Research Council, Australia (GNT1147442, GNT1123625, GNT1124356, GNT1100441, GNT1101533, GNT1100458) and several non-profit organizations. SA is a co-inventor on biomarker patents related to ferritin. RB is a full-time employee of Roche Diagnostics International. MMB is a full-time employee of Takeda Pharmaceuticals International Co. TB is a full-time employee of Genentech, a member of the Roche Group. JC has provided consultation to Acadia, Accera, Actinogen, ADAMAS, Alkahest, Allergan, Alzheon, Avanir, Axovant, Axsome, BiOasis Technologies, Biogen, Boehinger-Ingelheim, Eisai, Genentech, Grifols, Kyowa, Lilly, Lundbeck, Merck, Nutricia, Otsuka, QR Pharma, Resverlogix, Roche, Samus, Servier, Suven, Takeda, Toyoma, and United Neuroscience companies. JC receives support from Keep Memory Alize (KMA), COBRE grant #P20GM109025; TRC-PAD #R01AG053798; DIAGNOSE CTE #U01NS093334. AMF has served as a consultant or on advisory boards for AbbVie, Araclon/Grifols, DiamiR LLC, Eli Lilly, Genentech, IBL International, and Roche Diagnostics, and has received research support from Biogen, Fujirebio, and Roche Diagnostics. HH serves as Senior Associate Editor for the Journal Alzheimer’s and Dementia; he received lecture fees from Biogen and Roche, research grants from Pfizer, Avid, and MSD Avenir (paid to the institution), travel funding from Functional Neuromodulation, Axovant, Eli Lilly and company, Takeda and Zinfandel, GE-Healthcare and Oryzon Genomics, consultancy fees from Jung Diagnostics, Cytox Ltd., Axovant, Anavex, Takeda and Zinfandel, GE Healthcare Oryzon Genomics, and Functional Neuromodulation, and participated in scientific advisory boards of Functional Neuromodulation, Axovant, Eli Lilly and company, Cytox Ltd., GE Healthcare, Takeda and Zinfandel, Oryzon Genomics and Roche Diagnostics. HH is co-inventor in the following patents as a scientific expert and has received no royalties: (1) In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Patent Number: 8916388. (2) In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Patent Number: 8298784. (3) Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20120196300. (4) In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100062463. (5) In Vitro Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100035286. (6) In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Publication Number: 20090263822. (7) In Vitro Method for The Diagnosis of Neurodegenerative Diseases Patent Number: 7547553. (8) CSF Diagnostic in Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases Publication Number: 20080206797. (9) In Vitro Method for The Diagnosis of Neurodegenerative Diseases Publication Number: 20080199966. (10) Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20080131921. MMM served as a consultant to Eli Lilly and Lysosomal Therapeutics, Inc., and receives research support from the National Institutes of Health (R01 AG49704, P50 AG44170, U01 AG06786 RF1 AG55151), Department of Defense (W81XWH-15-1), and unrestricted research grants from Biogen, Roche, and Lundbeck. AM was an employee of Biogen when this manuscript was started. SO has served as a consultant to Roche Diagnostics and has multiple patents on blood biomarkers related to neurodegenerative disease. He is a founder of Cx Precision Medicine and owns stock. He receives research support for the NIH (R01AG058252, R01AG051848, R01AG054073) and the opinions discussed here do not reflect those of the NIA. PS has acquired grant support (for the institution) from Danone Research, Piramal and Merck. In the past 2 years he has received consultancy/speaker fees (paid to the institution) from Lilly, GE Healthcare, Novartis, Sanofi, Probiodrug, Biogen, Roche and EIP Pharma. JS was an employee of Roche when this manuscript was started. LMS receives research support from the National Institutes of Health (U19 AG024904, P30 AG-10124 for Biomarker Core), Eli Lilly, Hoffman LaRoche, MJFox Foundation for Parkinson’s Research for Biofind study and has served as consultant and/or advisory boards for Roche Diagnostics and Eli Lilly. HS is a full-time employee of AbbVie Inc. GT is a full-time employee of Lundbeck Pharmaceuticals LLC. JQT receives research support from the National Institutes of Health (P30 AG-10124-27, P01 AG-17586-18 and P50 NS-053488-11), Eli Lilly, Janssen, Biogen, and several non-profit organizations. JQT has received revenue from the sale of Avid to Eli Lily as co-inventor on imaging related patents submitted by the University of Pennsylvania, and may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is co-Inventor. HZ has served at advisory boards for Roche Diagnostics and Eli Lilly and has received travel support from Teva. KB has served as a consultant or at advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Merck, Novartis, Pfizer, and Roche Diagnostics.
Figures
References
-
- Alcolea D, Vilaplana E, Pegueroles J, Montal V, Sánchez-Juan P, González-Suárez A, et al. Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer’s disease. Neurobiol Aging. 2015;36:2018–2023. doi: 10.1016/j.neurobiolaging.2015.03.001. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

