Outcome for triple negative breast cancer in a retrospective cohort with an emphasis on response to platinum-based neoadjuvant therapy
- PMID: 30488345
- PMCID: PMC6418073
- DOI: 10.1007/s10549-018-5066-6
Outcome for triple negative breast cancer in a retrospective cohort with an emphasis on response to platinum-based neoadjuvant therapy
Abstract
Purpose: The rate of pathological complete response (pCR) for patients with triple negative breast cancer (TNBC) is increased when carboplatin is added to neo-adjuvant chemotherapy (NACT). However, while phase III trial data showing a survival benefit are awaited, carboplatin is not yet standard-of-care for TNBC. The aim of this study was to examine the rate of pCR and the outcome for those treated with carboplatin and to examine the predictors of response to therapy.
Methods: The retrospective series comprised 333 consecutive patients with TNBC (median follow-up time, 43 months). Adjuvant chemotherapy was given to 51% (n = 168) of patients and 29% (n = 97) received anthracycline-taxane NACT with carboplatin given to 9% (n = 31) of patients.
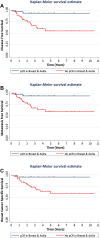
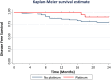
Results: Overall, 25% (n = 78) of patients experienced a breast cancer recurrence and 22% (n = 68) died from disease. A pCR breast and pCR breast/axilla was more common in those who received carboplatin (n = 18, 58% and n = 17, 55%, respectively) compared those who did not (n = 23, 36% and n = 18, 28%, respectively) (p = 0.041 and p = 0.011, respectively). By multivariable analysis, carboplatin and high tumor grade were independent predictors of pCR breast/axilla (ORnon-pCR = 0.17; 95% CI 0.06-0.54; p = 0.002; and ORnon-pCR = 0.05, 95% CI 0.01-0.27; p < 0.001, respectively). pCR breast/axilla was an independent predictor of DFS (HRnon-pCR=6.23; 95% CI 1.36-28.50; p = 0.018), metastasis-free survival (HRnon-pCR = 5.08; 95% CI 1.09-23.65; p = 0.038) and BCSS (HRnon-pCR = 8.52; 95% CI 1.09-66.64; p = 0.041).
Conclusion: Carboplatin therapy and high tumor grade are associated with a significant increase in the rate of pCR, which is an independent predictor of outcome. These data support the use of carboplatin in NACT for TNBC, while results from phase III studies are awaited.
Keywords: Breast cancer; Carboplatin; Neoadjuvant; Pathological complete response; Survival; Triple negative.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standards
This work complies with regulations governing ethical standards. Informed consent was obtained from patients who participated in this study, and the project was approved by the Clinical Research Ethics Committee, GUH (Ref. CA1012) on January 23, 2014.
Figures

References
-
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–4434. - PubMed
-
- Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. - PubMed
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. - PubMed
-
- Cheang MCU, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368–1376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

