Novel functions of peroxiredoxin Q from Deinococcus radiodurans R1 as a peroxidase and a molecular chaperone
- PMID: 30488429
- PMCID: PMC6590489
- DOI: 10.1002/1873-3468.13302
Novel functions of peroxiredoxin Q from Deinococcus radiodurans R1 as a peroxidase and a molecular chaperone
Abstract
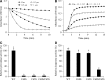
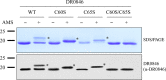
Deinococcus radiodurans R1 is extremely resistant to ionizing radiation and oxidative stress. In this study, we characterized DR0846, a candidate peroxiredoxin in D. radiodurans. DR0846 is a peroxiredoxin Q containing two conserved cysteine residues. DR0846 exists mainly in monomeric form with an intramolecular disulfide bond between the two cysteine residues. We found that DR0846 functions as a molecular chaperone as well as a peroxidase. A mutational analysis indicates that the two cysteine residues are essential for enzymatic activity. A double-deletion mutant lacking DR0846 and catalase DR1998 exhibits decreased oxidative and heat shock stress tolerance with respect to the single mutants or the wild-type cells. These results suggest that DR0846 contributes to resistance against oxidative and heat stresses in D. radiodurans.
Keywords: DR0846; Deinococcus radiodurans R1; molecular chaperone; peroxidase; peroxiredoxin Q.
© 2018 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures
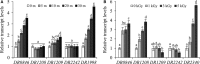


Similar articles
-
DRA0336, another OxyR homolog, involved in the antioxidation mechanisms in Deinococcus radiodurans.J Microbiol. 2010 Aug;48(4):473-9. doi: 10.1007/s12275-010-0043-8. Epub 2010 Aug 20. J Microbiol. 2010. PMID: 20799089
-
Site-specific mutagenesis of yeast 2-Cys peroxiredoxin improves heat or oxidative stress tolerance by enhancing its chaperone or peroxidase function.Protoplasma. 2017 Jan;254(1):327-334. doi: 10.1007/s00709-016-0948-0. Epub 2016 Feb 3. Protoplasma. 2017. PMID: 26843371
-
Engineering of 2-Cys peroxiredoxin for enhanced stress-tolerance.Mol Cells. 2011 Sep;32(3):257-64. doi: 10.1007/s10059-011-1047-x. Epub 2011 Jul 15. Mol Cells. 2011. PMID: 21773675 Free PMC article.
-
Gene regulation for the extreme resistance to ionizing radiation of Deinococcus radiodurans.Gene. 2019 Oct 5;715:144008. doi: 10.1016/j.gene.2019.144008. Epub 2019 Jul 27. Gene. 2019. PMID: 31362038 Review.
-
Antioxidative system of Deinococcus radiodurans.Res Microbiol. 2020 Mar;171(2):45-54. doi: 10.1016/j.resmic.2019.11.002. Epub 2019 Nov 19. Res Microbiol. 2020. PMID: 31756434 Review.
Cited by
-
Atypical Bacilliredoxin AbxC Plays a Role in Responding to Oxidative Stress in Radiation-Resistant Bacterium Deinococcus radiodurans.Antioxidants (Basel). 2021 Jul 20;10(7):1148. doi: 10.3390/antiox10071148. Antioxidants (Basel). 2021. PMID: 34356381 Free PMC article.
-
Relevance of peroxiredoxins in pathogenic microorganisms.Appl Microbiol Biotechnol. 2021 Aug;105(14-15):5701-5717. doi: 10.1007/s00253-021-11360-5. Epub 2021 Jul 14. Appl Microbiol Biotechnol. 2021. PMID: 34258640 Review.
-
Unraveling radiation resistance strategies in two bacterial strains from the high background radiation area of Chavara-Neendakara: A comprehensive whole genome analysis.PLoS One. 2024 Jun 10;19(6):e0304810. doi: 10.1371/journal.pone.0304810. eCollection 2024. PLoS One. 2024. PMID: 38857267 Free PMC article.
-
A Novel Thioredoxin-Dependent Peroxiredoxin (TPx-Q) Plays an Important Role in Defense Against Oxidative Stress and Is a Possible Drug Target in Babesia microti.Front Vet Sci. 2020 Feb 18;7:76. doi: 10.3389/fvets.2020.00076. eCollection 2020. Front Vet Sci. 2020. PMID: 32133382 Free PMC article.
-
The Construction of an Extreme Radiation-Resistant Perchlorate-Reducing Bacterium Using Deinococcus deserti Promoters.Int J Mol Sci. 2024 Oct 27;25(21):11533. doi: 10.3390/ijms252111533. Int J Mol Sci. 2024. PMID: 39519086 Free PMC article.
References
-
- Cox MM and Battista JR (2005) Deinococcus radiodurans – the consummate survivor. Nat Rev Microbiol 3, 882–892. - PubMed
-
- Ghosal D, Omelchenko MV, Gaidamakova EK, Matrosova VY, Vasilenko A, Venkateswaran A, Zhai M, Kostandarithes HM, Brim H, Makarova KS et al (2005) How radiation kills cells: survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress. FEMS Microbiol Rev 29, 361–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

